Abstract
Introduction
Hydrothorax is a rare complication of continuous ambulatory peritoneal dialysis (CAPD) which can progress quickly to cause acute respiratory distress.
Case presentation
We present a 76 year-old female with a past medical history significant for end-stage renal disease (ESRD) on daily home peritoneal dialysis for 2 years presented to the hospital from home with shortness of breath at rest and cough for 2 days prior to admission. She developed severe respiratory distress and had emergent pleurocentesis that released 3.8 L of pleural fluid. The analysis showed significantly high sugar indicative of hydrothorax from CAPD. She underwent thoracotomy with pleurodesis and switched to hemodialysis for 6 weeks before resuming CAPD.
Conclusion
A high glucose concentration in the pleural fluid is pathognomonic for hydrothorax from dialysis fluid after rule out other possible causes of pleural effusion. Patients who are on CAPD presenting with marked pleural effusion should prompt clinicians to consider the differential diagnosis of pleuroperitoneal communications.
Keywords: Hydrothorax, Pleural effusion, Peritoneal dialysis, Continuous ambulatory peritoneal dialysis, Pleuroperitoneal communication
Introduction
Continuous ambulatory peritoneal dialysis (CAPD) is commonly used in patients with end-stage renal disease (ESRD). Hydrothorax is a rare complication of CAPD. The infusion of dialysate fluid into the peritoneal cavity increases intraabdominal pressure which, along with negative intra-pleural pressure from spontaneous respiration, can cause peritoneal fluid to flow into the pleural cavity along pleuroperitoneal communications.
Case report
A 76 year-old female with a past medical history significant for end-stage renal disease (ESRD) on daily home peritoneal dialysis for 2 years, hypertension, hyperlipidemia, and osteoperosis, presented to the hospital from home with shortness of breath at rest and cough for 2 days prior to admission. She was in her usual state of health until 1 week prior to admission when she had mild shortness of breath after visiting her friend who was admitted to a hospital with pneumonia. Two days before admission, she had worsening shortness of breath and severe non-productive cough. She denied fever, chills, rigors, or nasal congestion. She had no history of trauma. She was prescribed azithromycin by a nephrologist without clinical improvement, prompting her visit to the emergency room.
On physical examination, blood pressure was 150/65 mmHg, heart rate was 84/minute, respiratory rate was 20/minute, with a body temperature of 97.8° Farenheit, with an oxygen saturation of 94% on 2 L by nasal canula. She was mildly pale in appearance. There was no jugular venous distension. Her cardiovascular examination was unremarkable. Breath sounds were decreased in the lower to mid lung zones on the right side, without wheezing or other adventitious sounds. Auscultation of the left lung was normal. Her abdomen was soft, and not tender, without organomegaly. A functional peritoneal dialysis line was present. She had no pedal edema. Her neurological exam was unremarkable.
Laboratory examination (Table 1) was significant for hemoglobin of 11.2 mg/dL, a white blood cell count of 14,000 cells/µL, (89% neutrophils, 4% lymphocytes, 6% monocytes, and 1% eosinophils), a platelet count of, blood urea nitrogen of 133 mg/dL, and a creatinine 6.94 mg/dL.
Table 1.
Laboratory data.
| Sources | Variable | Value | Unit |
|---|---|---|---|
| Blood | White blood cell count | 14,000 | cells/μL |
| Reb blood cell count | 3,660,000 | cells/μL | |
| Hematocrit | 34.3 | % | |
| Platelet count | 355,000 | cells/μL | |
| Segmented neutrophils | 89 | % | |
| Lymphocytes | 4 | % | |
| Blood urea nitrogen | 133 | mg/dL | |
| Creatinine | 6.94 | mg/dL | |
| Glucose | 135 | mg/dL | |
| Total protein | 7.1 | g/dL | |
| Albumin | 3.6 | mg/dL | |
| Lactate dehydrogenase | 49 | U/L | |
| Triglyceride | 58 | mg/dL | |
| Pleural fluid | Color | Colorless | |
| Appearance | Clear | ||
| pH | 7.63 | ||
| Glucose | 632 | mg/dL | |
| Total protein | <0.1 | g/dL | |
| Lactate dehydrogenase | 16 | U/L | |
| Triglyceride | <7 | mg/dL | |
| White blood cell count | 38 | cells/μL | |
| Red blood cell count | Few | cells/μL |
Her chest radiographs (Figs. 1 and 2) demonstrated a large right pleural effusion with associated atelectasis. Underlying pneumonia could not be excluded. There was also a small left pleural effusion.
Fig. 1.

Lateral chest radiograph at the time of admission.
Fig. 2.
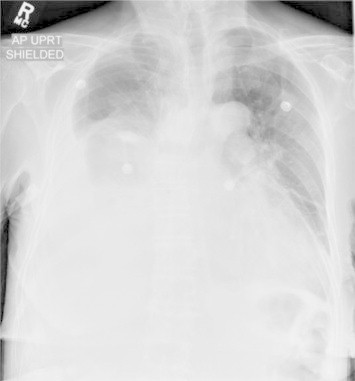
Anteroposterior (AP) chest radiograph at the time of admission.
The patient was admitted and treated for presumed healthcare associated pneumonia with vancomycin and ceftazidime, diuresis, supplementary oxygen and continued peritoneal dialysis. The next day, her clinical condition worsened, with oxygen saturation dropping to 91% on 6 L/minute by nasal canula. A repeat chest radiograph (Fig. 3) demonstrated worsening of the right pleural effusion. Emergent ultrasound-guided thoracocentesis was performed, and 3.8 L of colorless pleural fluid was drained. The patient had significant clinical improvement, with oxygen saturation increasing to 97% on 2 L/minute.
Fig. 3.
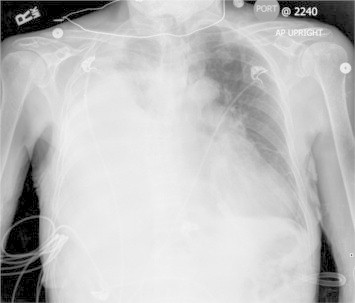
AP chest radiograph 12 hours after admission.
Pleural fluid analysis (Table 1) revealed clear fluid, 38 white blood cells/µL, few red blood cells/µL, pH 7.63, glucose 632 mg/dL (plasma glucose at that time 110 mg/dL), protein <0.1 g/dL (plasma protein 6.7 g/dL), triglyceride <7 mg/dL, no bacteria seen in gram stain and negative microbiological culture. Pleural fluid pathology showed no malignant cells.
CAPD was discontinued, and the patient received hemodialysis for treatment of her end-stage renal disease. A thoracotomy with pleurodesis was performed electively after discharge. She continued hemodialysis for 6 weeks before resuming daily peritoneal dialysis.
Discussion
Because of the high glucose concentration of the pleural fluid, this presentation was felt to be compatible with hydrothorax secondary to peritoneal dialysis. Cases of pleuroperitoneal communication have been previously described [1–3]. A high glucose concentration in the pleural fluid is pathognomonic for this condition, as no other etiology of hydrothorax has a marked elevation of glucose compared to serum levels [4].
Medical thoracoscopy is a well-tolerated procedure with an excellent diagnostic yield that is useful for patients with pleural effusion remaining underdiagnosed after initial pleurocentesis. Diagnostic yield was found to be more than 90% for a variety of conditions [5,6]. An isotopic peritoneogram with 99mTc-MAA was performed in one study to confirm the radiotracer activity at the right hemithorax [2]. A computed tomography of the Chest was performed after injecting radiocontrast medium mixed with peritoneal dialysis fluid intraperitoneally in order to identify the site of fistula [3]. Magnetic resonance imaging is another modality by which transdiaphragmatic leakage can be demonstrated [7].
Treatment modalities include temporary discontinuation of CAPD, along with either tube thoracotomy with tetracycline or talc slurry pleurodesis of the pleural space, surgical patch grafting of the diaphragmatic leak, or video-assisted talc poudrage.
Contribution
RRM acquired the data and drafted the article. MB revised the article critically for important intellectual content. RRM and MB approved the version to be submitted.
Acknowledgment
We would like to thank Saint Vincent Hospital staff for the patient's care.
References
- 1.Mahale A.S., Katyal A., Khanna R. Complications of peritoneal dialysis related to increased intra-abdominal pressure. Adv Perit Dial. 2003;19:130–135. [PubMed] [Google Scholar]
- 2.Garcia-Maldonado B., Guerrero-Ortiz M., Gomez-Fuente J.R., Garofano-Lopez R., Prados-Soler M.C., Del Pino Y., Pino M.D. Pleuroperitoneal communication in peritoneal dialysis patient. Nefrologia. 2012;32(4):551–552. doi: 10.3265/Nefrologia.pre2012.Jun.11489. [DOI] [PubMed] [Google Scholar]
- 3.Bae E.H., Kim C.S., Choi J.S., Kim S.W. Pleural effusion in a peritoneal dialysis patient. Chonnam Med J. 2011;47:43–44. doi: 10.4068/cmj.2011.47.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szeto C.C., Chow K.M. Pathogenesis and management of hydrothorax complicating peritoneal dialysis. Curr Opin Pulm Med. 2004;10:315–319. doi: 10.1097/01.mcp.0000127901.60693.d0. [DOI] [PubMed] [Google Scholar]
- 5.Menzies R., Charbonneau M. Thoracoscopy for the diagnosis of pleural disease. Ann Intern Med. 1991;114(4):271. doi: 10.7326/0003-4819-114-4-271. [DOI] [PubMed] [Google Scholar]
- 6.Sakuraba M., Masuda K., Hebisawa A., Sagara Y., Komatsu H. Diagnostic value of thoracoscopic pleural biopsy for pleurisy under local anaesthesia. ANZ J Surg. 2006;76(8):722. doi: 10.1111/j.1445-2197.2006.03839.x. [DOI] [PubMed] [Google Scholar]
- 7.Herbrig K., Reimann D., Kittner T., Gross P. Dry cough in a CAPD patient. Nephrol Dial Transplant. 2003;18:1027–1029. doi: 10.1093/ndt/gfg100. [DOI] [PubMed] [Google Scholar]


