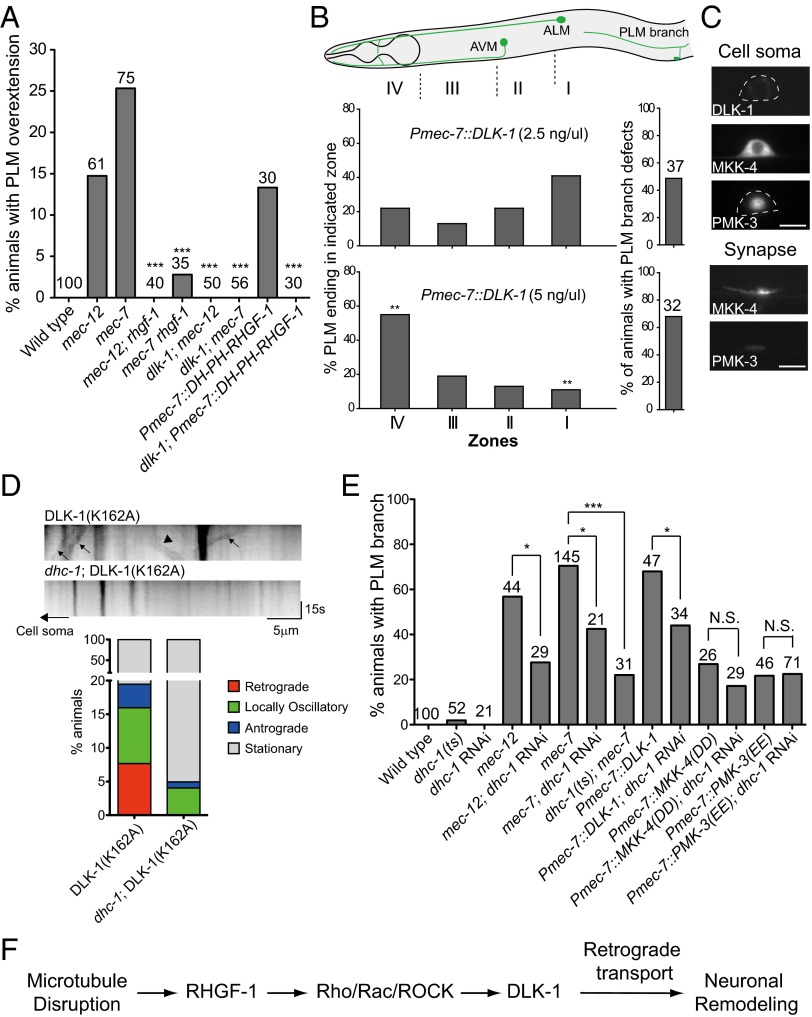
Fig. 4.
DLK-1 induced PLM branch defects and PLM process overgrowth. (A) Quantification of PLM neurite overextension in L4 larvae. DH-PH RHGF-1 transgenic animals were grown on plates with 0.125 mM colchicine. (B) PLM branch defects and neurite overextension in L4 larvae under different DLK-1 levels. Zones I–IV correspond to the termination sites of distal PLM process. **P < 0.005, two-proportion test. AVM, ; ALM, . (C) Localization of MAPK components in the PLM neuron of L4 larvae. (D) DLK-1(K162A) time-lapse imaging in L4 animals. (Upper) Representative kymographs of DLK-1 movements. Arrows and arrowheads mark representative DLK-1(K162A) puncta that moved in retrograde and anterograde directions, respectively. (Lower) Quantification of retrograde and anterograde movement events. (E) Effects of dhc-1 RNAi or dhc-1(or283) on PLM branch defects in L4 animals with the mec-7 mutation or excessive activity of DLK-1, MKK-4, or PMK-3. *P < 0.05; ***P < 0.001, two-proportion z test. mec-7 animals were reared at 25 °C after L1 and scored at L4 for PLM branch defects. N.S., not significant. (F) Mechanistic model of neuronal remodeling after microtubule disruption.