Fig. 3.
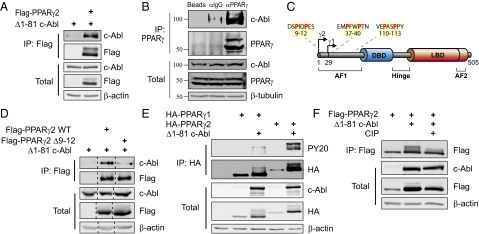
c-Abl binds and phosphorylates PPARγ2. (A) c-Abl binds PPARγ2. HEK293 cells were transfected with the indicated plasmids and analyzed by coimmunoprecipitation. (B) Endogenous c-Abl and PPARγ interact in adipocytes. 3T3-L1 cells were induced to differentiate. At day 5, cells were lysed; immunoprecipitated with either control empty beads, control anti-IgG–coated beads, or anti-PPARγ–coated beads; and protein levels were analyzed by Western blotting. The molecular weight of c-Abl is a bit lower than expected, which may suggest processing as has been previously reported (42, 43). (C) PxxP motifs within PPARγ; arrows denote transcription start sites for PPARγ1 and PPARγ2. The three PxxP motifs in the AF1 domain are highlighted. AF, activation function domain; DBD, DNA binding domain; LBD, ligand binding domain. Image modified from ref. 44. (D) The N-terminal PxxP motif of PPARγ2 is required for interaction with c-Abl. HEK293 cells were transfected with the indicated plasmids and analyzed by immunoprecipitation with anti-Flag beads. (E) c-Abl phosphorylates PPARγ2 but not PPARγ1. HEK293 cells were transfected with the indicated plasmids and analyzed by immunoprecipitation using anti-HA beads. Phosphorylation was detected by probing with an anti-phosphotyrosine antibody (PY20). (F) The mobility shift in the PPARγ2 band represents a phosphorylated form of PPARγ2. HEK293 cells were transfected with the indicated plasmids. Cell lysates were incubated in 37 °C for 30 min in the presence or absence of 150 U/mL calf-intestinal alkaline phosphatase (CIP) and were then analyzed by immunoprecipitation using anti-Flag beads.
