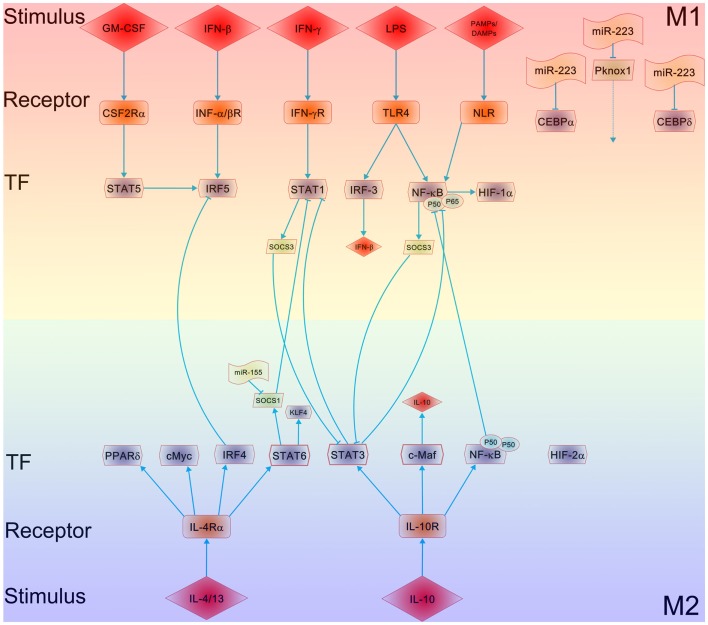
Figure 1.
Mechanisms underlying the polarization of macrophages. The major regulatory pathways of macrophage M1–M2 polarization are outlined. The crosstalk between the M1 and M2 macrophage polarizing pathways, particularly the balance between activation of STAT1 and STAT3/STAT6, tightly regulates macrophage polarization and activity. A predominance of NF-κB and STAT1 activation promotes M1 macrophage polarization, resulting in cytotoxic and tissue-damage proinflammatory functions. In contrast, a predominance of STAT3 and STAT6 activation by IL-4/13 and IL-10 increases M2 macrophage polarization, associated with immune tolerance and tissue repairing. PPARδ (and PPARγ) control distinct aspects of M2 macrophage activation and oxidative metabolism. KLF-4, a downstream of STAT6, participates in the promotion of M2 macrophage functions by suppressing the NF-κB/HIF-1α-dependent transcription. IL-4 induces not only c-Myc, which controls the expression of a subset of M2-associated genes but also the M2-polarizing IRF-4 axis to inhibit IRF5-mediated M1 polarization. IL-10 promotes M2 polarization through the induction of p50 NF-κB homodimer, c-Maf, and STAT3 activities. MicroRNAs such as miR-155, miR-223, etc. are involved in modulating macrophage polarization via targeting SOCS1, CEBP, and Pknox1, respectively.