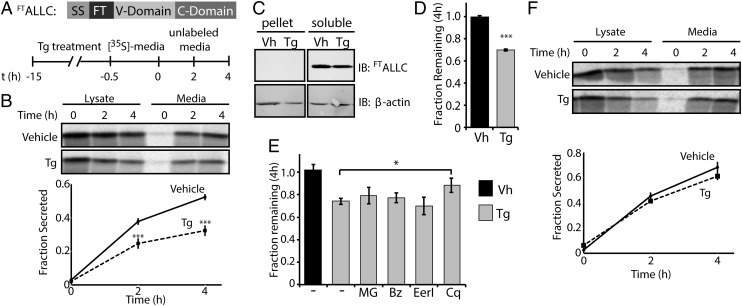
Fig. 2.
Thapsigargin selectively reduces the secretion of amyloidogenic LC and induces its degradation. (A) Schematic of the FTALLC construct used in pulse-chase experiments and the metabolic labeling protocol used. [35S]-labeled FTALLC was immunopurified from media and lysates collected from transfected HEK293T-Rex cells following a 15-h treatment with thapsigargin (Tg, 500 nM). (B) Representative autoradiogram and quantification of [35S]-labeled FTALLC in pulse-chase experiment described in A. Fraction secreted was calculated as described in Materials and Methods (18) (n ≥ 3). (C) Immunoblots measuring soluble and insoluble (pellet) levels of FTALLC after 15-h pretreatment with Tg. (D) Graph depicting total [35S]-labeled FTALLC (combined media and lysate protein levels as in B) remaining at 4 h in HEK293T-Rex cells following a 15-h pretreatment with 500 nM Tg (n ≥ 3). The fraction remaining was calculated as described in Materials and Methods (18). (E) Graph depicting the fraction recovery of total [35S]- labeled FTALLC at 4 h in HEK293T-Rex cells incubated in the presence of thapsigargin (Tg; 500 nM, 15 h) and the proteasome inhibitors bortezomib (Bz; 20 µM) or MG132 (MG; 20 µM), the ERAD inhibitor eeyarestatin I (EerI; 20 µM), or the autophagy inhibitor chloroquine (Cq; 100 µM). Inhibitors were incubated for 4 h before [35S] metabolic labeling and included throughout the labeling protocol shown in A. Fraction remaining was calculated as in D (n ≥ 2). (F) Representative autoradiogram and quantification of [35S]-labeled FTJTO immunopurified from media and lysates collected from transfected HEK293T-Rex cells following the same protocol and quantification as in A and B (n ≥ 3). *P < 0.05; ***P < 0.005. All error bars represent the SEM from biological replicates.