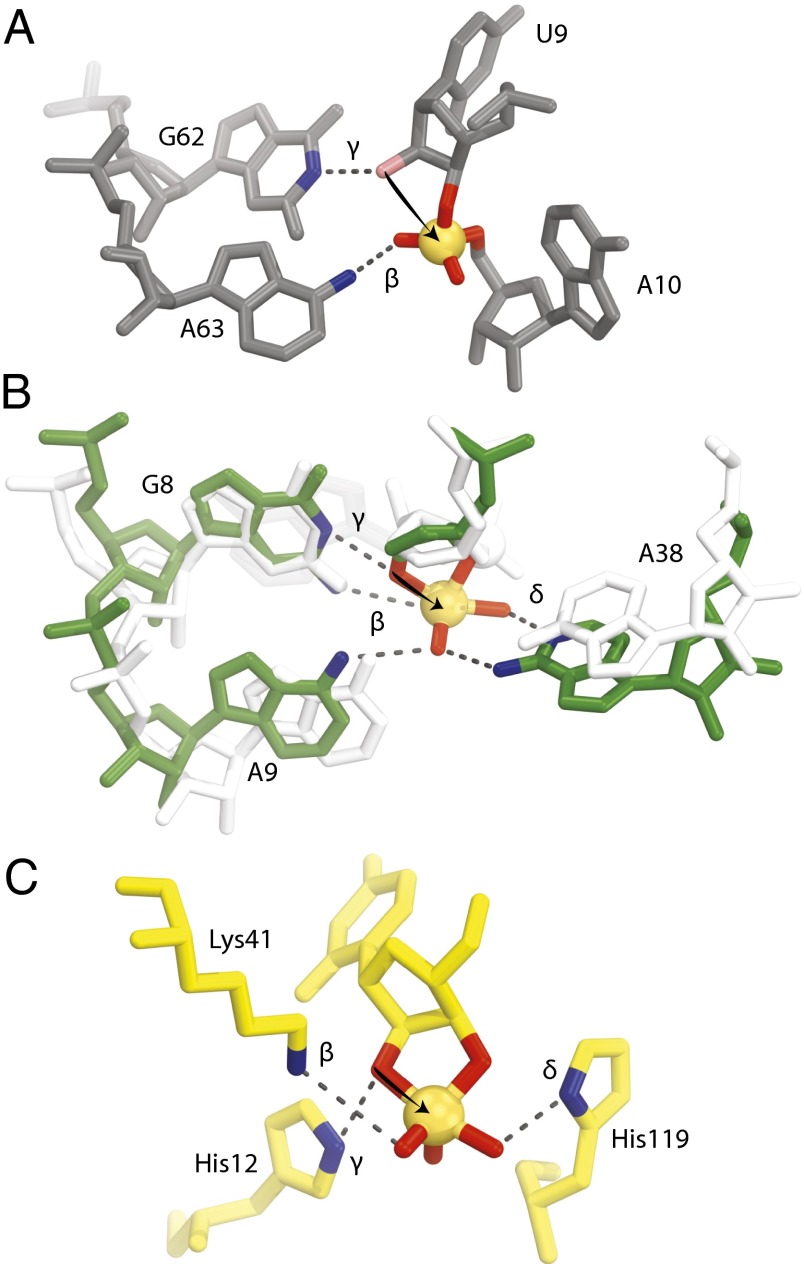
Fig. 4.
Comparison of the active sites for twister, hairpin, and RNase A. (A) The active site of twister with the conserved nucleotides suggested to be involved in catalysis are shown (environmental sequence). The 2′-hydroxyl is colored pink, and it was computationally modeled and not present in the crystallization construct. (B) Active site of hairpin precatalysis in white (1M5K) and transition state in green (1M5O). A9 and A38 move by about 2 Å each to cradle the phosphate, which also moves into position for in-line phosphoester transfer. (C) Active site of RNase A transition state (6RSA). The arrows are drawn to show the attack of the 2′-oxygen to the phosphate (orange spheres). Groups suggested to be involved in catalysis are shown with their proposed mechanistic strategies labeled. See Discussion for further explanation.