Abstract
Colorectal cancer (CRC) is one of the most common malignancies in the world and distant metastasis is the main cause of cancer-related mortality. Percutaneous computed tomography (CT) guided radioactive 125I seed implantation (CTRISI) is a minimally invasive technique used to treat pulmonary metastases in CRC patients. In the present study, following colorectal cancer resection, six patients with pulmonary metastases were treated with computed tomography (CT)-guided percutaneous implantation of radioactive 125I seeds. At six months following seed implantation, CT examination was performed and compared with the images captured prior to the treatment. Of the total 13 lesions, four had disappeared, eight were reduced by >50% and one was enlarged, indicating that the local control rate was 92.3% (12/13). Overall, two patients developed intraoperative pneumothorax and one experienced hemoptysis subsequent to the procedure. Following a median follow-up period of 31 months, no local recurrence was observed in 12 of the metastatic lesions. The mean survival time was 32.7 months and the median survival time was 31 months.
Keywords: colorectal cancer, pulmonary metastases, 125I seeds, computed tomography-guided
Introduction
Colorectal cancer (CRC) is the third most common malignancy in Western countries and is one of the leading causes of cancer-related mortality in China (1,2). In total, approximately 10–25% of patients with CRC develop pulmonary metastases (3). As no effective chemotherapy regimen has been developed for the treatment of pulmonary metastases of colorectal origin, surgery is the only potentially curative treatment option. However, only 2–4% of pulmonary metastases can be treated surgically and others require external beam radiotherapy and chemotherapy (4). Increasing the therapeutic doses of traditional external beam radiotherapy is challenging due to the severe side-effects. Although three-dimensional conformal radiation therapy (3D-CRT) and stereotactic external beam radiotherapy can administer tumoricidal doses, the side-effect of lung tissue damage remains a problem (5).
Percutaneous computed tomography (CT)-guided radioactive 125I seed implantation (CTRISI) is a minimally invasive modality. This brachytherapy is less time consuming and less traumatic, compared with the aforementioned treatments, and the side-effect of radiation damage is minimal (6). Patients are also more likely to accept this therapy due the minimally invasive nature of the technique. CTRISI has been used for the treatment of non-small cell lung cancer (NSCLC) (7,8). However, there have been few radioactive seed implantations for pulmonary metastases following resection of CRC and the efficiency of CTRISI has not been determined. The present study reports the preliminary results of six patients with pulmonary metastases following resection, who could not tolerate a surgical procedure and therefore, underwent CT-guided 125I brachytherapy.
Materials and methods
In total, six patients, three males and three females, with an ages range of 68–86 years (mean ± standard deviation, 76.0±7.6 years), with pulmonary metastases following colon cancer resection, were treated with percutaneous CTRISI at the Department of Thoracic Surgery, Second Hospital of Tianjin Medical University (Tianjin, China) between November 2002 and May 2010. Informed consent was obtained from the subjects and the present study was approved by the Ethics Committee of Tianjin Medical University. The patient characteristics are shown in Table I. Of the total 13 metastatic lesions, eight were located in the left lung and five in the right lung. In total, 10 were located in the lung and three were located beneath the hilum of the lung. The average diameter was 2.8±1.5 cm (range, 1–6 cm) and the average volume was 29.5±29.4 cm3. A complication of right supraclavicular lymph node metastasis was observed in one case and subsequently received seed implantation.
Table I.
Patient characteristics.
| Patient | Gender | Age, years | Time to recurrence, months | Adenocarcinoma pathology | Number of lesions | Location | Type | Diameter, cm | Volume, cm3 | Reason for seed implantation |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 71 | 4 | Poorly-differentiated | 1 | Adjacent to aortic arch | Central | 4 | 60 | Beside the aortic arch |
| 2 | Female | 71 | 12 | Well-differentiated | 1 | Lower right lung | Peripheral | 4 | 48 | Economic reasons |
| 3 | Male | 68 | 12 | Well-differentiated | 2 | Upper right lung | Peripheral | 2 | 10 | Multiple pulmonary metastases |
| Upper left lung | Peripheral | 2 | 15 | |||||||
| 4 | Female | 75 | 6 | Poorly-differentiated | 1 | Right pulmonary hilum | Central | 5 | 80 | Lymph node metastasis |
| 5 | Male | 85 | 36 | Well-differentiated | 2 | Right pulmonary hilum | Central | 6 | 96 | Multiple pulmonary metastases |
| Upper right lung | Peripheral | 2 | 10 | |||||||
| 2 | Upper left lung | Peripheral | 2 | 15 | ||||||
| Upper left lung | Peripheral | 3 | 27 | |||||||
| 1 | Lower left lung | Peripheral | 3 | 25 | ||||||
| 6 | Male | 86 | 45 | Well-differentiated | 3 | Upper left lung | Peripheral | 1 | 2 | Multiple pulmonary metastases |
| Upper left lung | Peripheral | 1 | 2 | |||||||
| Upper left lung | Peripheral | 2 | 8 |
The CT-guided brachytherapy procedure was carried out as previously described (9). Prior to the procedure, a treatment plan was prepared for each patient using a computerized treatment planning system (TPS; Prowess Panther, Prowess Inc., Concord, CA, USA) based on the CT images of the patients. The TPS generated a dose-volume histogram (DVH) and isodose curves of various percentages, and calculated the position (coordinates) of the brachytherapy applicator, dose and number of implanted seeds (Table II). Under local anesthesia, interstitial needles (Medical Device Technologies, Inc., Gainesville, FL, USA) were inserted into the tumor at ~0.5 cm intervals. Each 125I seed was implanted within an average of 1 cm3 of the tumor. The average planning target volume (PTV) of the 13 metastatic lesions was 32.1±30.1 cm3, and the average number of implanted seeds was 28±14.4 seeds. The average dose of the target area was 157.3±11.6 Gy, with a median dose of 152.4 Gy. The dose covered 90% of the volume (D90), 88.4±7.3 Gy, and the volume that received >90% of the prescribed dose (V90) was 31.5±29.5 cm3. Once the implant was completed, a CT scan was performed to verify the position and intensity of the 125I seeds according to TPS. The six-month post-procedural follow-up was complemented by CT examination. The follow-up was completed in May 2010 (Table III).
Table II.
Seed implant characteristics.
| Patient | Implantation time (yyyy/mm) | Location | PTV, cm3 | Planned number | Number implanted | Average dose, cGy | D90, cGy | V90, cm3 | Complication |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2002.11 | Upper left lung beside aortic arch | 64.6 | 35 | 35 | 14921.4 | 8880 | 63.3 | Pneumothorax |
| 2 | 2004.3 | Lower right lung | 53.1 | 34 | 35 | 16635.1 | 9200 | 50.8 | |
| 3 | 2005.3 | Upper right lung | 11.9 | 12 | 15 | 16923.3 | 9760 | 11.8 | |
| Upper left lung | 17.1 | 15 | 20 | 16390.9 | 9280 | 16.7 | |||
| 4 | 2005.7 | Right pulmonary hilum | 76.9 | 43 | 50 | 17659.8 | 9920 | 76.1 | Hemoptysis |
| 5 | 2007.8 | Right pulmonary hilum | 93.1 | 43 | 50 | 16532.1 | 8960 | 90.4 | Pneumothorax |
| Upper right lung | 10.8 | 8 | 10 | 15238.4 | 8206 | 10.3 | |||
| Upper left lung | 16.8 | 13 | 13 | 16253.9 | 8240 | 16.1 | |||
| Upper left lung | 32.1 | 28 | 27 | 15231.7 | 8170 | 31.8 | |||
| Lower left lung | 30.9 | 25 | 25 | 15085.5 | 7920 | 30.2 | |||
| 6 | 2008.5 | Upper left lung | 1.7 | 4 | 4 | 13822.4 | 8320 | 1.6 | |
| Upper left lung | 1.7 | 4 | 4 | 13822.4 | 8320 | 1.6 | |||
| Upper left lung | 8.4 | 10 | 10 | 16003.0 | 9920 | 8.4 |
PTV, planning target volume; D90, dose required to cover 90% of the volume; V90, volume that received >90% of the dose.
Table III.
Follow-up and outcome of the disease.
| Patient | Number of lesions | Location | Lesion status at six-month follow-up | Date of death (month/year) | Survival time, months |
|---|---|---|---|---|---|
| 1 | 1 | Adjacent to aortic arch | Decreased by 50% | 04/2005 | 29 |
| 2 | 1 | Lower right lung | Decreased by 50% | 04/2008 | 49 |
| 3 | 2 | Upper right lung | Disappeared | 08/2009 | 53 |
| Upper left lung | Disappeared | ||||
| 4 | 1 | Right pulmonary hilum | Enlarged | 03/2006 | 8 |
| 5 | 2 | Right pulmonary hilum | Decreased by 50% | 05/2010 | 33 |
| Upper right lung | Decreased by 50% | ||||
| 2 | Upper left lung | Decreased by 50% | 33 | ||
| Upper left lung | Decreased by 50% | ||||
| 1 | Lower left lung | Decreased by 50% | |||
| 6 | 3 | Upper left lung | Disappeared | 05/2010 | 24 |
| Upper left lung | Disappeared | ||||
| Upper left lung | Decreased by 50% |
Results
The brachytherapy catheters and 125I seeds were satisfactorily placed in all patients. Of the six patients, three developed pneumothorax during the procedure. These patients subsequently received chest-tube drainage as a curative treatment for the pneumothorax, and two to three days following this, the condition was resolved. Hemoptysis (~20 ml) was observed in one patient; this ceased two days following the oral administration of carbazochrome salicylate (5 mg three times a day, for three days).
Compared with the CT images captured prior to the procedure, the CT images obtained at the six-month follow-up revealed that four masses had been completely removed by the treatment and eight masses had been reduced in size by >50%. The CT images of one 86-year-old male patient prior to and following the procedure are shown in Figs. 1–5. Overall, only one mass was enlarged, indicating that the local control rate was 92.3% (12/13). None of the patients developed radioactive pneumonia or reduction in peripheral-blood granulocytes. Following a median follow-up period of 31 months (32.7±16.6 months; range, 8–53 months), no local recurrence was observed for the 12 metastatic lesions. Of the two patients with poorly-differentiated adenocarcinoma, one suffered from pulmonary metastases, complicated by right supraclavicular lymph node metastasis six months following radical resection. One of these patients succumbed to the disease eight months following brachytherapy and the other succumbed 29 months following brachytherapy. The four patients with well-differentiated adenocarcinoma succumbed to the disease 49, 53, 33 and 24 months following brachytherapy. The mean survival time was 32.7 months and median survival time was 31 months.
Figure 1.
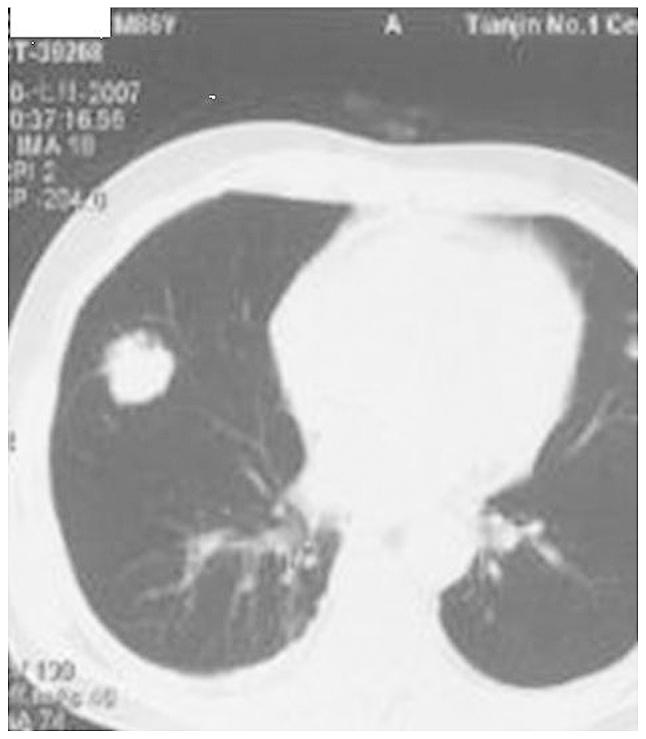
Male, 86 year-old patient 3 years following resection of colorectal cancer, revealing right lung metastases.
Figure 5.
Left pulmonary metastases reduced by >50% following 125I brachytherapy for six months.
Discussion
The present study demonstrates that percutaneous CTRISI is a feasible and promising, minimally invasive modality for controlling the growth of pulmonary metastases following CRC resection, particularly in the 12 months following surgery. Although one patient experienced hemoptysis and three patients suffered pneumothorax, these side-effects could be controlled, indicating that CTRISI remains a safe treatment method in this patient population.
The target area may tolerate sustained, closer high-dose irradiation through the conformal implantation of seeds into the interior of the tumor, overcoming target volume motion, so that the local control rate can be elevated. Independent or separate entity radiotherapy studies have demonstrated that local control of the tumor body is likely to be markedly intensified following irradiation with a bioeffect dose of 90–100 Gy. Martínez-Monge et al used CT-guided permanent brachytherapy to treat seven patients with early-stage T1N0M0 NSCLC. The median dose was 144 Gy. This study found that one patient developed a focal pneumonitis three months following the treatment, and no patients developed local or regional failure within a 13 month follow-up period (7). In the present study, the mean tolerance dose of PTV (gross tumor volume + 0.5 cm) was 157.3 Gy, with a median dose of 152.4 Gy. The lesions were irradiated with a dose of approximately twice the PD and a local control rate of 92.3% was achieved, which is similar to the effective rate of 93.8% (10) for seed implantation for pulmonary metastases and 87% (11) for 3D conformal external beam radiotherapy, as previously reported in the literature, demonstrating good therapeutic effects. This may be associated with the high-dose irradiation of the small target area as well as with the sensitivity of well-differentiated adenocarcinoma to γ-ray radiation.
The seeds can be accurately and evenly implanted into the target area under CT guidance. Patients with the target area located at the tumor center should undergo stereotactic puncture following contrast-enhanced CT scanning of blood vessels. The puncture needlepoint can be close to the heart and great vessels without injury to them. In the present study, D90 88.4 Gy (>PD) revealed a uniform and reasonable dose distribution.
The 125I-ray seed ray decays in an exponential manner with distance, which can reduce damage to tissues around the target area. The occurrence rate of radioactive pneumonia has been reported to be 44% (12) when the average therapeutic dose of the 3D conformal radiotherapy is 60 Gy. In the present study, no radioactive pneumonia was observed at PD 80 Gy, suggesting that seed implantation causes less damage as a result of radioactivity than conventional radiotherapy. Puncture-induced pneumothorax and hemoptysis can be observed on CT imaging and can be treated using conventional methods.
In conclusion, CTRISI is a safe and minimally invasive treatment modality for metastases from CRC that may aid in prolonging the survival rate in patients who cannot undergo pulmonary resection for metastases. While these results are promising, future studies including an increased number of cases, are required to gain further information with regard to CTRISI for the treatment of pulmonary metastases following CRC resection.
Figure 2.
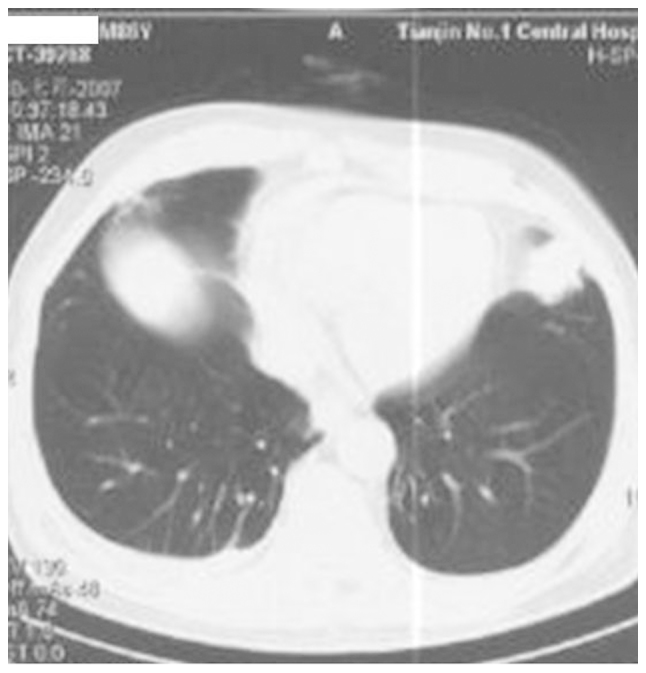
Male, 86 year-old patient 3 years following resection of colorectal cancer, revealing left lung metastases.
Figure 3.
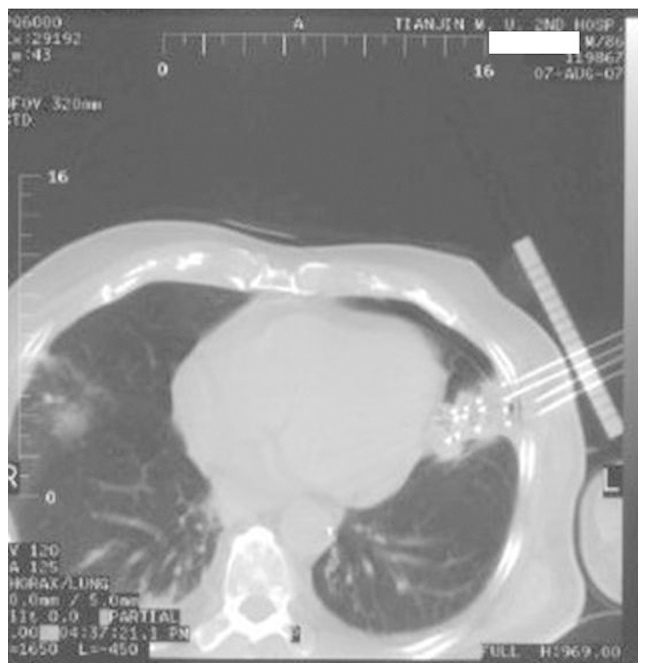
CT-guided 125I seed brachytherapy.
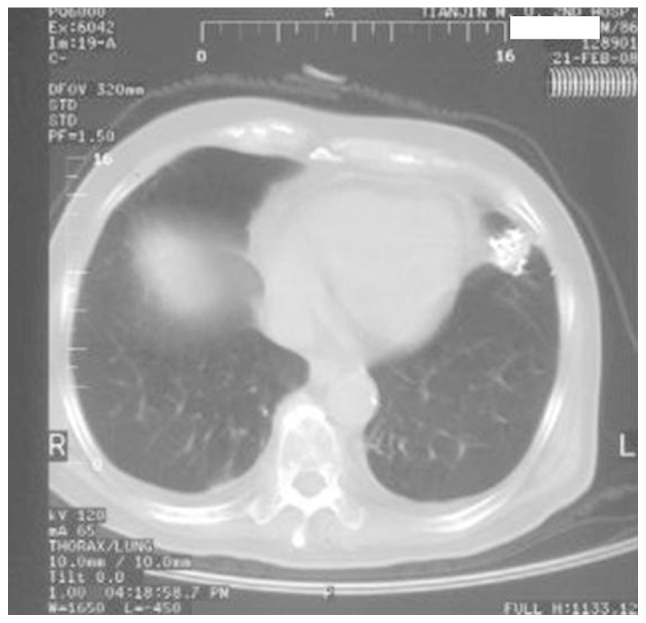
Figure 4.
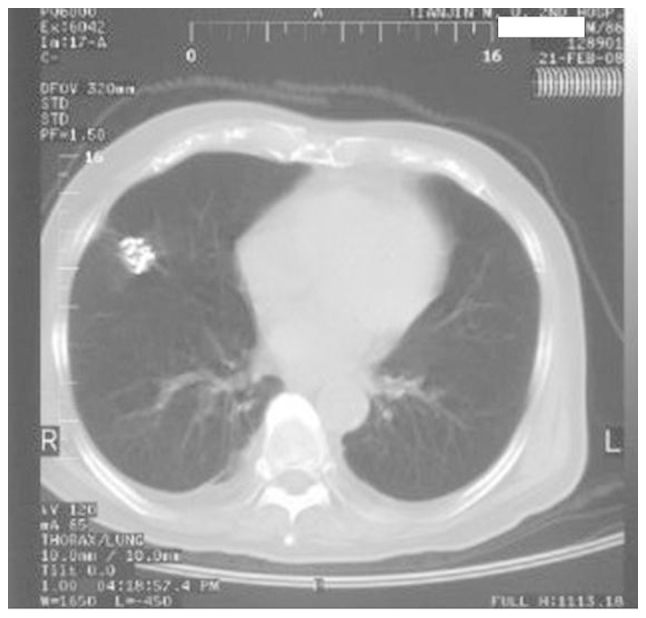
Right pulmonary metastases was reduced by >50% following 125I brachytherapy for six months.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2014 Sep 13; doi: 10.1002/ijc.29210. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 2.Talton BM, Constable WC, Kersh CR. Curative radiotherapy in non-small cell carcinoma of the lung. Int J Radiat Oncol Biol Phys. 1990;19:15–21. doi: 10.1016/0360-3016(90)90128-7. [DOI] [PubMed] [Google Scholar]
- 3.Rama N, Monteiro A, Bernardo JE, Eugénio L, Antunes MJ. Lung metastases from colorectal cancer: surgical resection and prognostic factors. Eur J Cardiothoracic Surg. 2009;35:444–449. doi: 10.1016/j.ejcts.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 4.Talton BM, Constable WC, Kersh CR. Curative radiotherapy in non-small cell carcinoma of the lung. Int J Radiat Oncol Biol Phys. 1990;19:15–21. doi: 10.1016/0360-3016(90)90128-7. [DOI] [PubMed] [Google Scholar]
- 5.Belderbos JS, Heemsbergen WD, De Jaeger K, Baas P, Lebesque JV. Final results of a Phase I/II dose escalation trial in non-small-cell lung cancer using three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:126–134. doi: 10.1016/j.ijrobp.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 6.Cha CM, Potters L, Ashley R, et al. Isotope selection for patients undergoing prostate brachytherapy. Int J Radiat Oncol Biol Phys. 1999;45:391–395. doi: 10.1016/S0360-3016(99)00187-X. [DOI] [PubMed] [Google Scholar]
- 7.Martínez-Monge R, Pagola M, Vivas I, López-Picazo JM. CT-guided permanent brachytherapy for patients with medically inoperable early-stage non-small cell lung cancer (NSCLC) Lung Cancer. 2008;61:209–213. doi: 10.1016/j.lungcan.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Zhang FJ, Li CX, Wu PH, et al. CT guided radioactive 125I seed implantation in treating localized advanced pulmonary carcinoma. Zhonghua Yi Xue Za Zhi. 2007;87:3272–3275. (In Chinese) [PubMed] [Google Scholar]
- 9.Martínez-Monge R, Garrán C, Vivas I, López-Picazo JM. Percutaneous CT-guided 103Pd implantation for the medically inoperable patient with T1N0M0 non-small cell lung cancer: a case report. Brachytherapy. 2004;3:179–181. doi: 10.1016/j.brachy.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Bradley J, Graham MV, Winter K, et al. Toxicity and outcome results of RTOG 9311: a phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:318–328. doi: 10.1016/j.ijrobp.2004.06.260. [DOI] [PubMed] [Google Scholar]
- 11.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 12.Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27:3290–3296. doi: 10.1200/JCO.2008.21.5681. [DOI] [PubMed] [Google Scholar]


