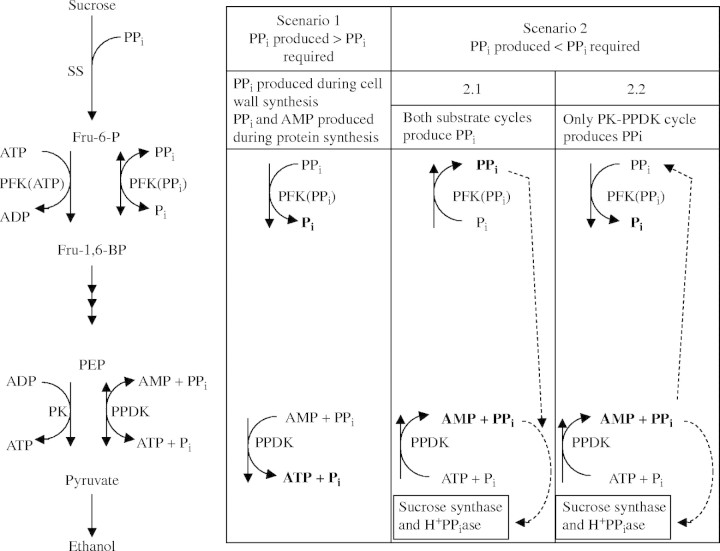
Fig. 6.
Possible role of pyruvate orthophosphate dikinase (PPDK) in rice coleoptiles in adaptation to anoxia. PPi could be produced or consumed by PPDK or pyrophosphate:fructose 6-phosphate 1-phosphotransferase (PFP). Two scenarios are possible; either PPi produced during polymer synthesis exceeded PPi required, or vice versa. Possible roles of PPDK and PFP during these two scenarios are shown. Scenario 1. PPi produced > PPi required. PPi produced during cell wall synthesis and protein synthesis allows a large glycolytic flux and economizes on energy spent during cell wall synthesis and amino acid activation. Scenario 2. PPi produced < PPi required, then the first possibility, scenario 2·1, is that both PPDK and PFP synthesize PPi for (a) sucrose conversion starting with sucrose synthase (SS) via UDP glucose pyrophosphorylase and for (b) tonoplast energization (via H+-PPiase). However, phosphofructokinase (PFK) would then have to catalyse the combination of the glycolytic flux and the flux of the substrate cycle flowing from PFK via F-1,6-bisP back to PFK, and in rice coleoptiles this may be well be above the capacity of PFK (see text and Noguchi, 2002). Hence we favor scenario 2·2: PPi would be synthesized by PPDK in a substrate cycle with pyruvate kinase (PK), while some of the PPi produced is used by PFP, allowing a large glycolytic flux to produce additional ATP for PPi synthesis in the PPDK substrate cycle.