Abstract
• Background Field observations and glasshouse studies have suggested links between boron (B)-deficiency and leaf damage induced by low temperature in crop plants, but causal relationships between these two stresses at physiological, biochemical and molecular levels have yet to be explored. Limited evidence at the whole-plant level suggests that chilling temperature in the root zone restricts B uptake capacity and/or B distribution/utilization efficiency in the shoot, but the nature of this interaction depends on chilling tolerance of species concerned, the mode of low temperature treatment (abrupt versus gradual temperature decline) and growth conditions (e.g. photon flux density and relative humidity) that may exacerbate chilling stress.
• Scope This review explores roles of B nutrition in chilling tolerance of continual root or transient shoot chills in crop species adapted to warm season conditions. It reviews current research on combined effects of chilling temperature (ranging from >0 to 20 °C) and B deficiency on growth and B nutrition responses in crop species differing in chilling tolerance.
•Conclusion For subtropical/tropical species (e.g. cucumber, cassava, sunflower), root chilling at 10–17 °C decreases B uptake efficiency and B utilization in the shoot and increases the shoot : root ratio, but chilling-tolerant temperate species (e.g. oilseed rape, wheat) require much lower root chill temperatures (2–5 °C) to achieve the same responses. Boron deficiency exacerbates chilling injuries in leaf tissues, particularly under high photon flux density. Suggested mechanisms for B × chilling interactions in plants are: (a) chilling-induced reduction in plasmalemma hydraulic conductivity, membrane fluidity, water channel activity and root pressure, which contribute to the decrease in root hydraulic conductance, water uptake and associated B uptake; (b) chilling-induced stomatal dysfunction affecting B transport from root to shoot and B partitioning in the shoot; and (c) B deficiency induced sensitivity to photo-oxidative damage in leaf cells. However, specific evidence for each of the mechanisms is still lacking. Impacts of B status on chilling tolerance in crop species have important implications for the management of B supply during sensitive stages of growth, such as early growth after planting and early reproductive development, both of which can coincide with the occurrence of chilling temperatures in the field.
Keywords: Boron deficiency, chilling temperature, photoinhibition, root hydraulic conductance, water channel
INTRODUCTION
Crop plants often experience multiple stresses due to the presence of many unfavourable growth conditions in the field, such as nutrient disorder, low temperature (both in the air and soil), soil acidity, salinity, drought and others. Among these adverse environmental factors, low temperature is a common threat to seedling establishment, flowering and seed/fruit set in many crop species, particularly those of tropical or subtropical origin (Lyons et al., 1979). In fact, for these species, chilling temperature (0–20 °C) has long been recognized as an important factor limiting the expansion of their production area (Lyons et al., 1979). Nevertheless, production areas of many crop species of tropical or sub-tropical origin that are prone to low temperature damage have been expanded into temperate regions for growth in the warm season. Boron (B) deficiency is the most widespread of all the micronutrient deficiencies in many crop regions from tropical to temperate zones (Shorrocks, 1997). Coincidently, these crop species such as sunflower, soybean and maize are also considered to be relatively high B-demanding species (Bell, 1997). As a result, in tropical, subtropical and temperate crop regions where low B soils are present, stresses resulting from chilling temperature and B deficiency are likely to be present simultaneously or sequentially.
Chilling can occur at low temperatures ranging from just above 0 to 20 °C, causing physiological dysfunction and damage without freezing or formation of ice in plant tissues (Lyons et al., 1979; Allen and Ort, 2001). The chilling sensitivity of crop species is assessed under defined conditions according to (a) the severity of chilling injuries in plant tissues, (b) the effectiveness of acclimation in preventing chilling injury at 5 °C, 85 % relative humidity (RH), and (c) the time for chilling injury to occur upon direct transfer from 25 to 5 °C at 100 % RH (Wise et al., 1983; Kratsch and Wise, 2000). For example, extremely chilling-sensitive species such as Gossypium hirsutum and Vigna radiata may show injured spots in leaf tissues after only 2 h at 5 °C and less, while intermediate chilling-sensitive species such as Cucumis sativum, Zea mays, Phaseolus vulgaris and Sorghum spp. show severe leaf injury only after 24 h of exposure to 5 °C under 85 % RH (Wise et al., 1983; Kratsch and Wise, 2000). Chilling-resistant species, such as oilseed rape and wheat, exhibit chilling injury in leaves only after prolonged exposure to the low end of the chilling temperature range under simultaneous high light conditions, but still maintain intact plastids in leaf cells and the capacity for regreening of leaf tissue before reaching advanced chilling injury (Kratsch and Wise, 2000).
Chilling temperature is one of the most studied environmental factors in plant stress physiology research. Since the first published report on tissue damage (chlorotic bands) on sugar cane leaves exposed to low night temperature (Faris, 1926), considerable progress was made in the 1970s and 1980s in understanding physiological impacts of chilling temperature in tropical and subtropical crop species (Lyons et al., 1979). Since the 1990s, research efforts have focused on molecular and sub-cellular events, in response to the primary actions of chilling temperature (e.g. Murata and Nishiyama, 1998; Melis, 1999; Kratsch and Wise, 2000; Allen and Ort, 2001; Browse and Xin, 2001). Comparatively recent research findings have also greatly improved our understanding of B uptake and transport processes (Brown and Shelp, 1997; Hu and Brown, 1997; Blevins and Lukaszewski, 1998; Brown et al., 2002a; Frömmer and von Wiren, 2002; Takano et al., 2002) and B roles in cell wall formation, cellular membrane functions and anti-oxidation defence systems (Cakmak and Römheld, 1997; Matoh, 1997; Goldbach et al., 2002). However, much remains to be understood about how B nutrition modulates plant chilling tolerance and how chilling tolerance affects plant responses to low/deficient B supply, particularly at the cellular level.
Continual root chilling over the short-term (several days) is probably more relevant to the field situation than continual shoot chilling, due to the high specific heat capacity of soil. Soils warm up much more slowly than do leaves and ambient air. In contrast, fluctuation of air temperature can be more transient and thus, transient shoot chills rather than the continual whole plant chill, are more likely to occur in the field. As a result, experimental findings of prolonged whole-plant chills should be interpreted with caution, in terms of their relevance to field situations. In the field, crops are often sown in late winter or early spring, with risks of prolonged root chilling during vegetative growth. Chilling in the root zone during early crop growth is likely to affect root growth and root system establishment and the uptake of B (and other nutrients) (Z. Ye, L. Huang and R. Bell, unpubl. res.). These effects may lead to low B status in leaves, which in turn may be exposed to transient chilling air temperature, thus lowering chilling tolerance of leaf cells, even though root chilling temperature may not be present when shoot chilling damage occurs. For the convenience of the following discussion, plant responses to root chills and shoot chills are dealt with separately. However, the pre-exposure of plants to either of the two stresses may have significant implications for the management of the other at different growth stages in the field.
This review is a discussion of the current understanding of chilling temperature and B nutrition interactions at the level of the organ and the whole plant. It explores possible primary events and processes in which B nutrition may interact with chilling temperature in plant tolerance responses, by focusing on: (a) how root chilling affects B uptake/transport and utilization efficiency; and (b) how pre-existence of B deficiency in the shoot influences chilling sensitivity of leaf cells, particularly in terms of photo-oxidative damage.
CHILL-INDUCED WATER RELATION RESPONSES AND B UPTAKE AND PARTITIONING
Most published studies have investigated effects of root zone chilling on plant responses to B by means of solution culture (e.g. Forno et al., 1979; Macduff et al., 1987; Ye et al., 2000, 2003). The intrinsic chilling sensitivity of crop species seems to be a determining factor in responses of B uptake/transport and B partitioning to root chills (Table 1). Crop species of tropical and subtropical origins are usually categorized as chilling-sensitive, ranging from extremely sensitive species such as Manihot esculenta (cassava), Ephedra vulgaris, Gossypium hirsutum and Vigna radiata, to moderately sensitive species such as Cucumis sativum, Glycine max, Zea mays, Phaseolus vulgaris and Sorghum spp. (Kratsch and Wise, 2000). Chilling resistant or tolerant species include Brassica spp., Hordeum vulgare, Triticum aestivum and Spinacia oleracea (Kratsch and Wise, 2000). In extremely chilling-sensitive cassava, exposure to root temperature of 18 °C for 28 d (shoots were exposed to ambient air temperature) at a relatively high B supply (46 μm B) in nutrient solution induced severe B deficiency symptoms initially in the roots and later in the shoot by the end of the experiment, due to the inhibition of B absorption by the root and its transport into the shoot. By contrast, plants grown at 22, 28 and 33 °C were free of B deficiency at the same level of B supply (Forno et al., 1979). In a less chilling-sensitive species, tomato (Lycopersicon esculentum ‘Burpee’) (Kratsch and Wise, 2000), reduction in B uptake at 93 μm B only occurred after root temperature was decreased to 10 °C (Tindall et al., 1990).
Table 1.
Effects of chilling root zone temperature on B nutrition in crop species of different chilling sensitivities (* the sensitivity ranking was based on Kratsch and Wise, 2000)
| Species |
Chilling sensitivity* |
Root temp. (°C) (day/night) |
Canopy temp. (°C) |
B supply (μm B) |
Chilling effects on B nutrition |
References |
|---|---|---|---|---|---|---|
| Cassava | Extreme | 18 | Ambient | 46 | Severe B deficiency symptoms in roots first and then in shoots later. Decreased B absorption rate. Increased shoot : root ratio | Forno et al., 1979 |
| Sunflower | Intermediate | 12 | Ambient | 0·08, 0·16, 0·25, 0·62, and 6·5 | Induced B deficiency symptoms in young leaves at 0·25 μm (but not at 17 °C and above), decreased B uptake at all B supply levels, B transport from root to shoot, B partitioning into young leaves. Increased shoot : root ratio | Ye et al., 2000 |
| Tomato | Intermediate | 10 | Ambient | 93 | No B deficiency symptom, decreased B absorption by roots | Tindall et al., 1990 |
| Wheat | Tolerant | 5 (night only) | 5 (night only) | 0, 10 | No B deficiency symptom, decreased B concentrations in the youngest emerging leaf (flag leaf), but increased B concentration in the ear at 10 μm B and decreased at 0 μm B. | Huang et al., 1996 |
| Oilseed rape | Tolerant | 10 | Ambient | 0·13, 0·19, 8·3 | Decreased shoot : root ratio, delayed B deficiency symptoms at 0·13 μm B, increased shoot B partitioning into actively growing leaves without effect on net B uptake rate, and increased B concentration in the youngest fully opened leaves | Ye et al., 2003 |
| Oilseed rape | Tolerant | 2–5 | Ambient | 0·2, 9·1 | Direct transfer from 10–12 to 2–5 °C in root zone for 5 d decreased water use (per plant), increased shoot : root ratio, B uptake rate, B translocation into shoot, and B partitioning into the new leaves, regardless of B supply. | Ye, 2005 |
However, exposure to 10 °C in the root zone did not decrease B uptake in chilling-tolerant species, such as oilseed rape and wheat (Huang et al., 1996; Ye et al., 2003), because this temperature is above their chilling threshold (Macduff et al., 1987). In the chilling-tolerant winter crop oilseed rape, root growth is tolerant to low root temperature, leading to an increased root : shoot ratio at 10 °C in the root zone temperature (Kacperska and Szaniawski, 1993). Nevertheless, B uptake rate, B translocation from root to shoot and B partitioning into the actively growing young leaves of oilseed rape plants were significantly decreased after abrupt transfer from 10–15 to 2–5 °C in the root zone and under high light conditions (mid-day photon flux density: 1500–2000 µmol m−2 s−1) during the day (Ye, 2005). However, such an abrupt root temperature decline may not necessarily occur in the field.
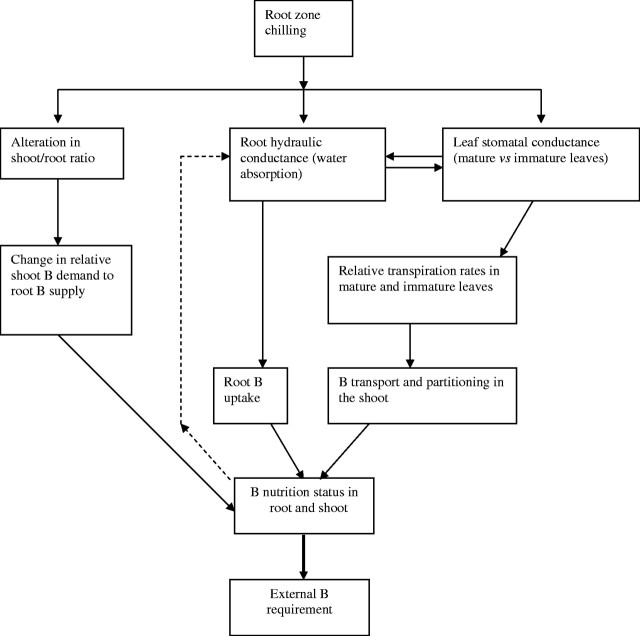
Species/genotypic variation in chilling tolerance is closely related to the effects of chilling on root water absorption and leaf transpiration (Capell and Dörffling 1993; Allen and Ort, 2001; Aroca et al., 2001; Bloom et al., 2004). As a result, the responses in B uptake/transport and partitioning in the shoot by chilling-sensitive and -tolerant species (Table 1) may be related to chilling effects on plant–water relationships: including root water absorption, root hydraulic conductance and leaf stomatal responses (Fig. 1). In addition, B uptake across the plasma membrane by permeation through the lipid membrane and water channels may be greatly influenced by plant chilling tolerance through the associated changes in root hydraulic conductivity, particularly when root B supply falls into the ‘active uptake’ range which is around 1 μm B in soil solution for a range of species (Dannel et al., 2000; Asad et al., 2001). However, the underlying physiological mechanisms by which plant responses to root chilling modulate B uptake and transport are yet to be experimentally established, let alone the relevant molecular processes at the cellular/subcellular levels.
Fig. 1.
A conceptual diagram relating root chill-induced changes in plant–water relationships (root hydraulic conductance, leaf stomatal conductance and water potential) and associated effects on B uptake, transport and partitioning.
Root hydraulic conductance
In chilling-sensitive species such as cucumber, tomato and sunflower, chill-induced water loss is one of the most significant physiological consequences of root chilling, resulting from decreased root hydraulic conductance and excessive transpiration due to dysfunction of stomatal control (delayed closure or closure failure) (Allen and Ort, 2001). In fact, stomatal closure and/or maintaining root hydraulic conductance (defined as the steady-state root exudation rate under specified root pressure) and water absorption are important mechanisms of acclimation to root chills in chilling-tolerant genotypes of the same species (Capell and Dörffling, 1993; Aroca et al., 2001, 2003b; Bloom et al., 2004). The signalling mechanisms of stomatal closure in response to root chills were initially attributed to the rapid production and accumulation of abscisic acid (ABA) in roots and leaves (such as in maize; Capell and Dörffling, 1993), but subsequent studies suggest that there is no clear causal relationship between ABA contents in root and leaf stomatal conductance among genotypes of different chilling tolerance (Aroca et al., 2003b; Bloom et al., 2004).
The decrease in root hydraulic conductance caused by root chilling would have significant consequential and coupled effects on root B uptake and B transport from root to shoot (Fig. 1). At adequate B levels, root B uptake is mostly a passive process of permeation of undissociated boric acid across the plasma membrane, which is largely determined by the rate of water uptake through the plasma membrane of root cells (Hu and Brown, 1997), plus the flow through water channels (Dordas et al., 2000). In contrast, recent studies argue for active uptake mechanisms when external B supply is low (e.g. below 1 μm B) (Dannel et al., 2000, 2002). In chilling-sensitive species like cassava, sunflower and tomato, root chilling-induced reduction of B absorption rate and enhanced B deficiency (Table 1) are likely to be caused by decreased root hydraulic conductance and water absorption, apart from the direct effect of chilling on root absorption surface area and the root : shoot ratio. However, at similar root zone temperature (10–15 °C), chilling-tolerant species, such as oilseed rape, are capable of maintaining high root hydraulic conductance and root growth (Aroca et al., 2001), which may explain why root chills did not decrease the net B uptake rate in oilseed rape plants at 10 °C in the root zone, compared with 20 °C (Ye et al., 2003).
It is important to consider the treatment method used and environmental conditions such as photon flux density and canopy temperature, when interpreting any chilling-enhanced sensitivity to B deficiency in crop species. Despite the chilling tolerance of oilseed rape, prolonged exposure under high vapour deficit conditions (high irradiance and canopy temperature, low relative humidity) to the lower end (2–5 °C) of the chilling temperature range decreased B uptake by roots, B translocation from root to shoot and B partitioning into young leaves (Ye, 2005). In contrast, a gradual decrease (2 °C h−1) in root temperature to 6 °C induced root acclimation and decreased electrolyte leakage from leaf tissues, compared with those plants abruptly transferred from 20 to 6 °C (Ahn et al., 1999).
Role of water channels
In plants, 75–95 % of the water moving across the plasma-membrane passes through water channels (Steudle, 2000). According to the composite model, parallel apoplastic, symplastic and transcellular pathways all contribute to the process of water movement across different tissues of the root cylinder (epidermis, exodermis, central tracheary elements), but the contribution of water channels in the plasma membrane to water uptake by root cells increases under conditions of environmental stresses, such as drought and nutrient depletion (Steudle and Peterson, 1998).
Water channels in the plasma membrane of root cells play an important role in root hydraulic conductivity and plant–water relationships, particularly in response to changing environmental factors (e.g. drought, nutrient deficiency, low temperature) (Tyerman et al., 1999, 2002). Regulation of the expression of specific water channel proteins in plasma membranes can change the cellular osmotic water permeability coefficient up to 3-fold (Kaldenhoff et al., 1998). Wheat root hydraulic conductance is greatly reduced by limiting N and P supply, probably due to the reduced activity or abundance of Hg-sensitive water channels in the roots (Carvajal et al., 1996). However, it is yet to be established if B deficiency could lower the activity and/or abundance of water channels in the plasma membrane though its role in membrane integrity and function.
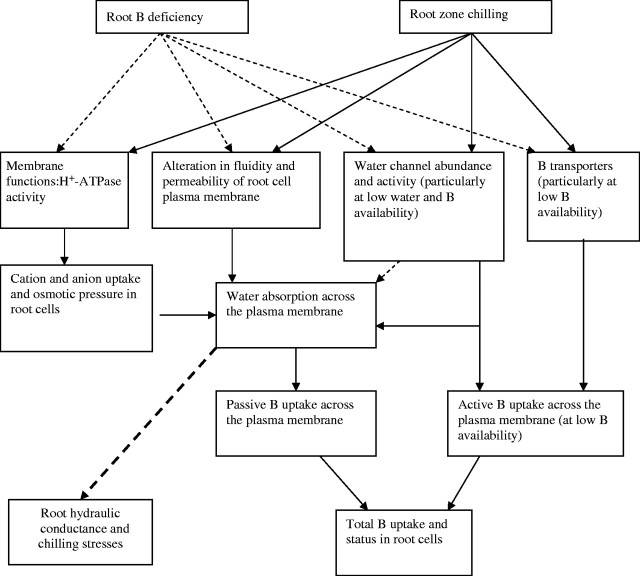
In tomato plants root-chilled at 5 °C, root hydraulic conductance reduction declined nearly twice as much as the calculated value based on decreased water viscosity alone (Bloom et al., 2004). This evidence suggests that apart from the reduction in water permeation across the plasma membrane, the ‘gating’ or ‘closing’ of water channels in chilling-sensitive species is highly likely to occur in response to root chills (Fig. 2). In rice (Oryza sativa ‘Josaeng Tongil’), the expression of a novel aquaporin gene in root cells is correlated with root acclimation to chills and chilling-tolerance (Li et al., 2000). In addition, blocking water channels (through altering protein conformation) by the non-specific agent HgCl2 lowered the chilling tolerance of a chilling-tolerant maize genotype, due to the associated inhibition of root hydraulic conductance (Tyerman et al., 1999; Aroca et al., 2001). Water channels in the plasma membrane may be also reversibly closed by hydroxyl radicals (*OH) (Henzler et al., 2004). Coincidently, the net accumulation of oxidative free radicals (including *OH) in cells is also one of the consequences of B deficiency in root and leaf cells (Cakmak and Römheld, 1997).
Fig. 2.
A conceptual diagram about possible mechanisms involved in the interaction between chilling stress and B deficiency in root cells: membrane permeability and functions (water channels and proton-pumping ATPase).
Water channels (or aquaporins) also play an important role in the process of B uptake across the plasma membrane (Dordas and Brown, 2000; Dordas et al., 2000). The B permeability coefficient (3 × 10−7 cm s−1) of purified plasma membrane vesicles from squash roots was six times higher than that of microsomal vesicles and B permeation across the plasma membrane vesicles was reduced by 30–39 % by the addition of the non-specific channel blocking agent—HgCl2 (Dordas et al., 2000). The contribution of channel-mediated B uptake to the total root B uptake may be particularly significant when external B concentrations are low but still above the active B uptake range. As a result, chilling stress in the root may result in an increased apparent external B requirement, leading to a higher risk of B deficiency during the period of seasonal transition from cold to warm temperature when air warms up much faster than the soil, such as in late-winter and early spring.
The postulated involvement of water channels in the uptake of boric acid may be particularly relevant at low external B supply, under conditions of root chills (Fig. 2). This may be due to: (a) the strong association between passive B uptake and water absorption across the root cylinder (Hu and Brown, 1997); (b) the role of water channels in the permeation of boric acid across the plasma membrane of root cells (Dordas et al., 2000); and (c) the rapid response of water channels to gating signals sensing environmental stresses (Tyerman et al., 1999, 2002). For example, the rise in ABA levels in roots and leaves is one of the rapid responses to root chilling in maize, in the process of chilling acclimation (Capell and Dörffling, 1993; Aroca et al., 2003b; Bloom et al., 2004). The ABA in root cells specifically increases the activity of water channels, water permeability of cell membranes and hydraulic conductivity in the root (Steudle, 2000).
On the other hand, the pre-existence of B deficiency in young roots may enhance the sensitivity of roots to chilling (Fig. 2). Severe B deficiency (e.g. interruption of B supply to the root tips for 1 h) causes a rapid decrease in the amount of the plasma membrane water channel proteins (ZmPIP1 aquaporins) in the root apex of maize and transgenic tobacco (Goldbach et al., 2002). This impact may lower the ability of roots to maintain hydraulic conductivity in response to chilling stress, even in chilling-tolerant species or genotypes. Experimental evidence is required to test this hypothesis. However, it is important to note that the complete depletion of B from soil solution would rarely happen due to the buffering capacity of soils, except perhaps on very sandy soils under intense leaching rainfall. Hence, the extreme conditions of complete depletion of external B may not simulate plant responses in soils. Experimental systems, such as B-buffered solution culture, are required to maintain realistically low solution B concentrations (Huang et al., 1999).
Boron loading into the xylem
Boron loading into the xylem after passive uptake into root cells requires the function of B transporters (similar to a B transporter in animal cells; see Park et al., 2004) located in the plasma membrane of the root pericycle, particularly when external B levels are low (Takano et al., 2002). The recently suggested mechanism of active B uptake and transport in roots at low B supply (Dannel et al., 2000, 2002) may be related to the functions of these B transporters (Takano et al., 2002). Since the activity of B-transporters is metabolically dependent and hence sensitive to low temperature, chilling temperature may inhibit active B transport across the pericycle into the stele of the root cylinder at low B supply.
Plasma membrane function and permeability
Chill-induced changes in the function and integrity of root cell plasma membranes may also contribute to the associated decreases in water and nutrient (including B) uptake by plant roots. Such changes include cell membrane lipid composition (Lyons et al., 1979), membrane fluidity (Queiroz et al., 1998) and dysfunction of membrane-bound enzymes (e.g. H+-ATPase; Shabala and Newman, 1997; Ahn et al., 1999). In chilling sensitive species, cellular membrane alterations precede other cellular changes, and adverse effects on different cellular organelles are dependent on duration of chilling and associated growth conditions including photon flux density and relative humidity (Lyons et al., 1979).
Dordas and Brown (2000) demonstrated that different proportions of sterols and longer chain fatty acids in the plasma membrane of root cells significantly changed B uptake in Arabidopsis thaliana mutants, and related these changes to different permeability coefficients for boric acid across the plasma membrane containing different groups of lipids and fatty acids. Decrease in sterol content in the plasma membrane may increase membrane fluidity and permeability to water and ions (Lyons et al., 1979), which is correlated with plant chilling tolerance (Hugly et al., 1990). Increased membrane rigidity is a common response to chilling temperature in chilling-sensitive species such as Coffea arabica (Queiroz et al., 1998). As a result, chilling-induced reduction in membrane fluidity and permeability of root cells may also contribute to the inhibition of B uptake in chilling-sensitive species (Fig. 2).
Proton pumping, one of the key plasma membrane functions, exhibits a rapid response to root chills (within minutes). An important root acclimation process in cucumber plants is that low temperature (6 °C) increases the rate of the H+-transport of the plasma membrane and the amount of plasma membrane H+-ATPase gene transcription and translation (Ahn et al., 1999). The response time required for ‘switching on’ the active H+-efflux pumping seemed to be related to the general chilling tolerance of the species tested: 4 min for the chilling-tolerant pea (Pisum sativum) and bean (Vicia faba); and 11 min for the chilling-sensitive cucumber (Cucumis sativus) and pumpkin (Cucurbita pepo) (Shabala and Newman, 1997). Root chilling at 5 °C for only 2 h significantly decreased NH4+ absorption in tomato plants (Bloom et al., 1998). The post-chilling recovery of the kinetics of cation (e.g. K+, Ca2+, NH4+) and anion (e.g. Cl−, NO3−) fluxes across the plasma membrane of root cells seems to be determined by the critical chilling temperatures for ion transporters of plant species (Pettersson, 1995; Bloom et al., 1998; Shabala and Shabala, 2002). For chilling-tolerant species like oilseed rape and barley, the net uptake of NH4+ by roots recovered much faster from a chilling episode than did net NO3− uptake, but the opposite was true for the chilling-sensitive species like tomato (Macduff and Wild, 1989; Macduff and Jackson, 1991; Smart and Bloom, 1991). Comparatively, root chills suppress the uptake of cations more than anions (Shabala and Shabala, 2002). This higher sensitivity of cation than anion uptake to root chills may be related to the sensitive responses of the plasma membrane H+-ATPase and the H+-gradient across the plasma membrane, which is the direct driving force for cation transport across the plasma membrane into the cytoplasm (Marschner, 1995). Chilling-induced rapid effects on the uptake of cations may also indirectly affect root hydraulic conductance through solute-accumulation and increases in osmotic potential of root cells (Fig. 2).
Rapid responses to B deficiency in the function and integrity of the plasma membrane of root cells have been well established (see reviews by Cakmak and Römheld, 1997; Goldbach et al., 2002). For example, the activity of the plasma membrane H+-ATPase and NADH+-reductase can be inhibited within several minutes of B deficiency treatment, lowering the net H+ efflux and causing the failure of hyperpolarization of the plasma membrane. This B deficiency-induced inhibition of H+-ATPase activity causes a decrease in cation uptake, such as K+ (Schon et al., 1990). Solute uptake and accumulation in root cells are important in the establishment of osmotic potential gradients in the root cylinder and its overall hydraulic conductivity (Steudle, 2000). As a result, B deficiency in roots may further enhance the sensitivity of root cells to chilling stresses by reducing the uptake of other cations and ions that are dependent on the H+-pumping function of the plasma membrane ATPase.
The plasma membranes of root cells may also be damaged by oxidative free radicals generated by chilling or B-deficiency stress. Root chilling at 10 °C caused lipid peroxidation and associated increased membrane rigidity in root cells of Coffea arabica (Queiroz et al., 1998). Boron deficiency in the root has been known to decrease the level of antioxidants such as the reduced form of ascorbate (Lukaszewski and Blevins, 1996; Cakmak and Römheld, 1997). Oxygen free radicals in root cells would be formed in the process of root respiration in the mitochondria and the oxidation of secondary metabolites such as soluble phenols (Prasad et al., 1994; Queiroz et al., 1998). However, root respiration tends to decrease with decreasing root temperature (Szaniawski and Kielkiewicz, 1982). As a result, it is assumed that chilling-induced oxidative damage in root plasma membrane would be a secondary consequence of prolonged stress, in comparison to sensitive responses of the plasma membrane to B deficiency. In any case, such oxidative damage in root cells would be much less acute than oxidative damage in leaf cells, where, in addition to chilling air temperature, cells have to cope with reactive oxygen species generated from photosynthesis (see below).
Stomatal response and B partitioning
Leaf stomatal response to root chills may be a major factor influencing shoot B use efficiency. Root chills decrease B partitioning into new leaves, and thus increase the sensitivity of leaf growth to low B supply (Forno et al., 1979; Tindall et al., 1990; Ye et al., 2000). From the available experimental evidence (Table 1), root chill-induced effects on B transport into, and B partitioning within, the shoot may be regulated by chilling-induced leaf stomatal responses and transpiration, particularly in mature leaves, which are expected to contribute to most of the transpiration activity in the canopy on the basis of their leaf area. In chilling-sensitive species like cassava, sunflower and tomato, root chills decreased B uptake rates, B transport from root to shoot, and B partitioning into young leaves, consequently enhancing the sensitivity of new shoot growth to B deficiency, at low/marginal external B supply (e.g. at 0·25 μm B in sunflower at 12 °C) (Forno et al., 1979; Tindall et al., 1990; Ye et al., 2000). In contrast, low root temperature (10 °C) delayed B deficiency in chilling-tolerant species like oilseed rape compared with 20 °C in the root zone (Ye et al., 2003), through maintaining root growth and B uptake rate and increasing B partitioning into new shoot growth. The difference in leaf stomatal control between chilling-sensitive species and tolerant species at marginal temperatures such as 10 °C may explain their different responses in chilling-induced B deficiency sensitivity at about the same root temperature (10–12 °C), though 10 °C was above the threshold temperature for oilseed rape roots. This suggestion requires further experimental evidence by measuring stomatal conductance in the leaves of different age in test plants (such as sunflower and oilseed rape) over a time-course following exposure to low B and chilling temperatures in the root zone.
Both root and shoot chilling can cause a reduction in leaf stomatal conductance in a range of species, such as bean (Phaseolus vulgaris) (Matzner and Comstock, 2001), maize (Aroca et al., 2003a, b) and tomato (Bloom et al., 2004). However, realistically, chilling air temperature is more likely to occur during the night when leaf transpiration is minimal; whereas continual root chills are more likely to be persistent for days, during which air temperature around the shoot fluctuates during the day.
In chilling-sensitive species, chilling decreases root hydraulic conductance and slows down water absorption on the one hand, and on the other hand, impairs stomatal control which leads to excessive water loss and leaf wilting (Capell and Dörffling, 1993; Bloom et al., 2004). Chilling-tolerant maize lines were found to have lower transpiration and higher water potentials in the recently matured leaves than those in chilling-sensitive lines (Capell and Dörffling, 1993). Stomata remained open in a chilling-sensitive tomato cultivar (Lycopersicon esculentum ‘T5’) when root temperature declined to 5 °C, but not in a chilling-tolerant cultivar (‘LA 1778’) (Bloom et al., 2004). After 30 h exposure to 5 °C, a chilling-tolerant maize genotype acclimated by increasing root hydraulic conductance to the control level after an initial decline in root hydraulic conductance, whereas the sensitive genotype maintained low root hydraulic conductivity and higher leaf transpiration (Aroca et al., 2001). As a result, chilling tolerance involving leaf stomatal control would play an important role in B use efficiency in the shoot and B requirements for plant growth.
Chilling-induced changes in shoot hydraulic conductance and the relative transpiration intensity of mature and immature leaves will not only affect the total B transport into the shoot, but also the partitioning of B into immature leaves where there is the greatest B functional demand in the shoot. After B uptake into root cells and loading into the xylem, the transport of B from root to shoot is largely driven by transpiration in the leaves, and the distribution of B in the shoot follows the gradient of transpiration rate in leaves of different age (Brown and Shelp, 1997; Huang et al., 2001). Partitioning of B into the immature leaves (or enclosed ears in the case of cereal species) faces a strong competition from the sink-strength of mature leaves that have much higher transpiration activity (Huang et al., 2001). Suppression of transpiration and therefore B-sink strength in mature leaves by increasing relative humidity around the shoot significantly increased the proportion of B partitioned into the young ear with minimal transpiration activity because of its enclosure by the flag leaf sheath, with minimal transpiration activity (Huang et al., 2001). Conversely, any chilling-induced failure in stomatal closure in expanded leaves may contribute to higher transpiration and B-sink strength in mature leaves relative to immature ones, leading to reduced B partitioning into the young leaves, such as in the case of chilling-sensitive sunflower subject to 12 °C in the root zone (Ye et al., 2000). The consequence of stomata remaining open in mature leaves would be a lower B concentration in the youngest mature leaves and a greater sensitivity to B deficiency at low B supply, compared with those at 22 °C (Ye et al., 2000).
In summary, root chilling would have a profound impact on B uptake by the root and transport towards the new growth in the shoot, particularly in chilling-sensitive species such as sunflower, which coincidently have a high-B demand (Bell, 1997). As a result, an increased external B supply may be necessary for these crops in the field when there is a high risk of root chills in the soil. This extra B supply to the root may help counter the decreased B diffusion towards the root surface due to the increased water viscosity, and the reduced B uptake rate and transport due to chill-induced decline in root hydraulic conductance and failure in leaf stomatal regulation in the leaves. The minimization of B deficiency in roots (particularly in the early growth phase) may, in turn, alleviate chilling-induced water deficit by increasing root absorption surface area and maintaining adequate water and nutrient uptake by root cells.
BORON IN CHILLING-INDUCED PHOTOINHIBITION
Chronic and marginal B deficiency has been reported in many crop species across large areas of field production (Shorrocks, 1997). Field observations have suggested a link between chilling canopy temperature and enhanced leaf tissue damage (bleached patches) in oilseed rape grown in low B soil in south-east China (Ye, 2005), though further soil trials in the glasshouse and/or field conditions are necessary to produce conclusive evidence about links between B and leaf chilling tolerance. In a subtropical woody species, chilling-induced ‘white top’ (bleached young leaves) has been frequently observed in chilling-sensitive Eucalyptus urophylla clones in south China (X. Daping, pers. comm.) where B-deficient soils are common (Dell and Malajczuk, 1994), but a direct link between B and chilling-induced leaf damage was not confirmed in these field cases. In a controlled experiment, exposure to 5 °C significantly increased the electrolyte leakage in the young leaves of Eucalyptus urophylla (subtropical species) at low B supply (5 μm B), but not at adequate B (15 μm B) (Lu and Huang, 2003). These reports have led to the suggestion that suboptimal or deficient B status in the leaves (particularly young and recently matured leaves) may reduce chilling-tolerance of leaf cells, but the proposition needs to be further supported with direct experimental evidence. The following section focuses on possible physiological and biochemical mechanisms by which B deficiency could modulate chilling tolerance in relation to photosynthesis in leaf cells.
Important factors to be considered in the interpretation of photosynthetic responses to chilling temperature are canopy conditions such as photon flux density, air temperature and relative humidity, which can greatly alter plant sensitivity to chilling (Kratsch and Wise, 2000). In particular, increasing photon flux density can lower plant tolerance to chilling (Wise et al., 1983). For oilseed rape grown in glasshouse conditions under high photon flux density (1500–2000 µmol m−2 s−1 during 1100–1500 h), root chilling at 2–5 °C for 5 d significantly decreased root growth, B uptake by roots, B translocation from root to shoot and B partitioning into new shoot growth, enhancing plant sensitivity to B deficiency, in comparison with responses at 10 and 15 °C in the root zone (Ye, 2005). As a result, plant growth conditions should be clearly defined when comparing chilling responses of different test species and their interaction with B deficiency in different experiments.
Photo-oxidation and chilling-tolerance
Chilling temperature around leaves can disrupt key processes in photosynthesis, including thylakoid electron transport, carbon assimilation and stomatal control (Kratsch and Wise, 2000; Allen and Ort, 2001). The severity of chilling stress on photosynthesis can be particularly exacerbated by simultaneous or sequential exposure to light due to the enhanced risks of photo-oxidative damage (Allen and Ort, 2001). In Phaseolus vulgaris, quantum yield was severely decreased by exposure to 6 °C at 2000 µmol m−2 s−1 for 3 h, but not at 12 °C or under low photon flux density (Powles and Critchley, 1980). In one of the most chilling-sensitive species, Gossypium hirsutum (Kratsch and Wise, 2000), leaf chlorosis was not observed after 144 h exposure to chilling (5 °C) in the dark though severe leaf wilting appeared after 72 h, but under 500 µmol m−2 s−1 light, the same temperature caused leaf chlorosis after 48 h and permanent wilting of the fully expanded leaves by 24 h (Wise et al., 1983). Ultrastructurally, chilling in the light for 144 h caused the loss of starch granules and dilation of thylakoids in chloroplasts but not in the dark (Wise et al., 1983). This photosynthesis-dependent chilling tolerance may be modified by B deficiency due to B roles in plasma membrane integrity and functions (Cakmak and Römheld, 1997) and in cellular redox balance and the oxidative repairing system (Kobayashi et al., 2004).
On the basis of very limited information, B deficiency induced a much greater sensitivity to shoot chilling than root chilling, in terms of gross structural changes (e.g. membrane leakage) and photosynthetic functions of leaf cells (Ye, 2005), even in a chilling-tolerant species such as oilseed rape (Wise et al., 1983). In a chilling-sensitive cucumber cultivar, chilling temperature (7–8 °C/5 °C, day/night, under 300 µmol m−2 s−1 light) enhanced membrane leakage (K+) induced by B-deficiency and chloroplast disruption and plasmolysis of mesophyll cells (Wang et al., 1999). Chilling-temperature around leaves induced a higher solute leakage in the youngest open leaf of low B-status oilseed rape (10–15 mg B kg−1 dry matter) than leaves containing adequate B (25 mg B kg−1 dry matter) (Ye, 2005). This gross structural damage may be the consequence of chronic photo-inhibition caused by chilling stress in leaf cells with low/deficient B status, and the further effects of high light flux density (Huang et al., 2002).
Chilling-induced reduction in photochemical reaction (CO2 assimilation) and in inhibition of thylakoid electron transport may be the fundamental causes for photoinhibition at chilling air temperature (either in the dark or in the light), including dynamic (repairable) and chronic (photodamage caused by oxidative radicals) photoinhibition (Allen and Ort, 2001). The primary event of photoinhibition is suggested to be the functional inactivation of the photosystem II (P680) reaction centre (Cleland, 1988), where the steady-state oxidation-reduction level of the primary quinone acceptor (QA) of PS II must be maintained to avoid photo-oxidation of the light harvesting centre (Melis, 1999). The extent of PS II photoinhibition is closely related to the redox state of the electron acceptor QA in PS II and the level of reduced QA increases with light flux density, leading to increased risks of photodamage in PS II (Melis, 1999; Sonoike, 1999). When thylakoid electron transport is inhibited through the normal pathway of CO2 assimilation, excitation energy stored in the reduced QA at PS II is dissipated via a charge-recombination reaction with oxygen (rather than CO2 fixation), which generates singlet oxygen (Melis, 1999). The highly reactive oxygen free radicals O2−* and OH* on the acceptor side of PS II, which increase with photon flux density levels (Arato et al., 2004), may consequently cause photo-oxidation of chlorophyll and chloroplastic membranes (Melis, 1999). These oxidative effects caused by chilling may be further exacerbated by B deficiency, as the latter induces a rapid oxidative response in the cytoplasm of tobacco cells within 30 min of low B treatment (Kobayashi et al., 2004).
Plant chilling tolerance will depend on the intrinsic anti-oxidation systems in leaf cells, which may be weakened by B deficiency (Lu and Huang, 2003). Due to the limitation of direct experimental evidence in the literature, the following discussion focuses on photo-oxidative processes in leaf cells, in which chilling and B nutrition are most likely to interact with each other, by reviewing current knowledge about their separate effects on photo-oxidative responses.
Boron deficiency is likely to enhance chilling-induced photoinhibition in leaf cells, through its possible effects on photosynthesis. Kastori et al. (1995) observed that relatively prolonged exposure (23 d) to B deficiency (1 μm B) significantly decreased photosynthetic oxygen generation in leaves, quantum yield and PS II efficiency, which was attributed to reduced electron transport efficiency and the accumulation of soluble carbohydrates in leaves. In a follow-up study by the same group (Plesnicar et al., 1997), B deficiency was found to reduce the maximum rate of photosynthetic O2 production, but had no effect on the efficiency of PS II electron transport. The reduction in maximum quantum yield may be related to the reduction of chlorophyll content and photochemical quenching of electrons (Plesnicar et al., 1997). In sunflower at 0·02 μm B, the decline in oxygen generation rate and non-photochemical quenching in leaves accompanied a range of effects on other metabolic processes which include accumulation of sucrose and phenolic compounds and stimulated peroxidase activity and lipid peroxidation (El-Shintinawy, 1999). The existing evidence of B deficiency-induced effects on leaf photosynthesis has been mostly obtained from long-term (10 d or longer) treatments of plants with deficient B supply. These long-term B-deficiency effects on leaf photosynthesis make it hard to pinpoint the primary events induced by B-effects on thylakoid membrane, photochemistry or electron transport efficiency within the chloroplast.
One of the possible mechanisms by which B deficiency affects photosynthetic responses to chilling may be through B roles in the integrity and functions of thyalkoid and chloroplast membranes. Boron deficiency rapidly affects the functions of enzymes and other proteins in the plasma membrane, transport processes across the membrane and membrane integrity (Cakmak and Römheld, 1997; Brown et al., 2002b; Goldbach et al., 2002). Rapid effects of B deprivation on plasmalemma enzyme functions in plant cells are well known (see reviews by Cakmak and Römheld, 1997; Blevins and Lukaszewski, 1998; Brown et al., 2002b; Goldbach et al., 2002). Boron deficiency reduces the activity of proton-pumping ATPase and thus the proton gradient across the plasma membrane, and inhibits oxidoreductase in the plasma membrane within minutes of B deprivation (Goldbach et al., 1991; Barr et al., 1993). Both ATP and NADPH are products of photochemical electron transport in response to light reaction in chloroplasts (Melis, 1999). These early effects of B deficiency on the plasma membrane may also be present in chloroplastic membranes including the thylakoid membrane, which may consequently lower the overall efficiency of electron transport through PS II and PS I, leading to the oxidative damage to the membrane, such as lipid peroxidation as observed in sunflower leaves (El-Shintinawy, 1999).
The pre-exposure of leaf cells to suboptimal B nutrition may also lower the activity of anti-oxidation systems and decrease the possibility of recovery from photoinhibition in the photosystems after chilling stress. So far, there are no data to support any direct role for B in enzymes involved in plant anti-oxidation systems. However, B deficiency can decrease the levels of antioxidants in leaves, including ascorbic acid (reduced form), SH-compounds and glutathione reductase (Cakmak and Römheld, 1997). The decrease in reduced-ascorbate level in leaves seems not to be a cause for B deficiency-induced membrane leakage, which is rapidly reversed by B addition into the incubation solution of sunflower leaf discs (Pfeffer et al., 1998). In leaves of a subtropical Eucalyptus grandis × E. urophylla hybrid, B deficiency treatments (0 and 5 μm B) for up to 96 h at 5 °C significantly increased the production rate of superoxide (O2−) and polyphenol oxidase activity, but not in plants with adequate (15 μm) B supply at the same temperature (Lu and Huang, 2003). Lu and Huang (2003) also found that the activities of anti-oxidation enzymes (superoxide dismutase, peroxidase, catalase and ascorbate peroxidase) in leaves were decreased at 5 °C with B deficiency (<10 μm B), but not at 15 μm B.
As a result, B deficiency may increase the sensitivity of leaf cells to chilling, through enhanced generation of oxidative free radicals and weakened anti-oxidation capacity. However, published findings about B in photosynthesis were mostly made at the organ or tissue level, which make the differentiation of causal events occurring at subcellular level (e.g. chloroplast membrane, thylakoid membrane) difficult, due to inconsistency in leaf cell age, leaf cell water potential (transpiration) and B status within the same organ or tissue. We may ask whether a B role in photo-oxidation is the consequence of primary effects of B in chloroplast membrane integrity and functions or the result of metabolic process failure such as substrate feed back and suppressed anti-oxidation activity in the chloroplast membrane. To achieve defined physiological and biochemical responses in leaf cells to B deficiency and/or chilling stress without the complications of the above inconsistency, protoplast or/and chloroplast culture may be used to explore the proposed mechanisms in relation to B roles in photosynthesis under chilling treatments over a time course.
Although the B-deficiency-induced phenolic oxidation initially proposed by Marschner (1995) and Cakmak and Römheld (1997) has been shown to be a secondary effect of B function in membrane integrity (Cara et al., 2002), the accumulation of soluble phenolics and subsequent oxidation in leaf cells may give rise to permanent damage to the cells and leaf tissue death, leading to reduced photosynthetic area. Phenolics accumulate in B-deficient leaf tissues due to enhanced phenylalanine-ammonium lyase (PAL) activity, particularly in high B-demanding species like sunflower and tobacco (Marschner, 1995; Ruiz et al., 1998), which are also chilling sensitive (Kratsch and Wise, 2000). The initial loss of membrane integrity induced by B deficiency provides the opportunity for phenolic (stored in vacuoles) oxidation catalysed by polyphenol oxidase located in the thylakoid membrane and in cell walls (Pfeffer et al., 1998; Cara et al., 2002). Coincidently, chilling also enhances the production of phenolic compounds in plant cells, through increasing the level of PAL due to an inducible imbalance in PAL enzyme synthesis and its degradation (relatively lower degradation rate) (Graham and Patterson, 1982). Increasing light flux density stimulates the biosynthesis of phenolics (Mole et al., 1988; Chattopadhyay et al., 1994), due to the light-induced activity of PAL (Bolwell and Butt, 1983). As a result, photo flux density may also influence the secondary accumulation of phenolics in leaves of suboptimal B status while being exposed to chilling temperature.
Chilling temperature increases superoxide dismutase (SOD) activity, leading to increased production of H2O2, that with superoxide (O2−), forms highly reactive OH− radicals in leaf cells (Saruyama and Tanida, 1995). Chilling tolerance in plant species is closely related to the capacity of anti-oxidation systems in leaf cells, which consist of oxidative free-radical scavenging enzymes [e.g. SOD, ascorbate peroxidase (APX) and catalase (CAT)] and anti-oxidants (e.g. ascorbate, glutathione and β-carotene). In one of the chilling-sensitive species, cucumber, chilling leaves at 5 °C in the light caused an increase in H2O2 concentration in leaves, due to a substantial decline (by up to 80 % compared with that at 25 °C) in the activity of the thylakoid APX which is a key enzyme in H2O2-scavenging (Terashima et al., 1998). The chilling tolerance of cucumber seedling radicles correlated positively with the level of APX and CAT activity, but negatively with activity levels of SOD, glutathione reductase and guaiacol peroxidase (Kang and Saltveit, 2002). In inbred maize lines, tolerance to chilling stress increased with the levels of antioxidants in leaves, including ascorbate and β-carotene (Hodges et al., 1996). Photo-oxidative conditions induced by chilling in the light can cause a range of ultrastructural distortion in chloroplasts, including thylakoid dilation and chloroplast swelling (Kratsch and Wise, 2000).
In summary, it is postulated that B deficiency may exacerbate chilling-induced photo-oxidative damage and weaken the anti-oxidative system for photo-inhibition recovery in leaf cells, through (a) enhanced generation of oxidative free radicals, at least through B-deficiency-induced disturbance to enzyme functions (such as H+-ATPase and NADP-reductase) in thylakoid membranes and/or B-deficiency induced substrate feedback—inhibition of triose-phosphate export and starch accumulation in chloroplasts, and (b) weakening the recovery capacity from photo-oxidative damage by decreasing enzyme activities in oxidative free-radical metabolism and the levels of antioxidants (e.g. ascorbate).
CHILLING ACCLIMATION AND CELL WALL COMPOSITION: IMPLICATION IN INTERNAL B REQUIREMENT
Boron cross-linking with the pectic polysaccharide, rhamnogalacturonan II (RG-II), plays a crucial role for stabilizing primary cell walls (Matoh, 1997; Brown et al., 2002b; Goldbach et al., 2002). The role of B in cell wall structure is the cause of the positive correlation between internal B requirements of different plant species and the amount of pectin (as a percentage of cell walls) (Hu et al., 1996). If genetically determined chilling tolerance has any bearings on cell wall composition in terms of the percentage of pectin in the cell wall, it would be expected that chilling-tolerant genotypes may have higher B requirements for cell growth than sensitive genotypes.
Acclimation of winter oilseed rape at 2 °C for 3 weeks substantially increased the contribution of cell walls to leaf dry matter and the content of pectin in the cell walls, but the increase in cell wall pectin was largely due to the increase of non-covalently bound pectins [ammonium-oxalate extractable and KOH (0·1 m) extractable pools] (Kubacka-Zebalska and Kacperska, 1999). It remains to be established if leaf cell walls of chilling-tolerant genotypes or warm-climate species may have higher covalently bound pectins and more importantly the proportion of B-binding polysaccharide RG-II in the cell walls.
CONCLUDING REMARKS AND FIELD IMPLICATIONS
In summary, chilling in the root zone impairs plant–water relationships through chilling-induced reduction of root hydraulic conductance and the loss of stomatal control (leading to water deficit in leaves) in chilling-sensitive species such as coffee, mung bean and cucumber (Aroca et al., 2001; Bloom et al., 2004) and, at the cellular level, impairs membrane structure and function in root cells (Shabala and Newman, 1997; Ahn et al., 1999; Shabala and Shabala, 2002). Because in most crop species, B is passively taken up by roots, transported in the xylem and deposited towards sinks with the highest transpiration rate (Brown and Shelp, 1997; Hu and Brown, 1997), chilling effects on root water absorption and hydraulic conductance in roots and shoots may suppress root B uptake and transport, and alter B partitioning into new shoot growth (as observed by Ye et al., 2000, 2003). Molecular approaches may be applied to reveal primary events involved in the complex interaction between chilling temperature and B deficiency on the activity and abundance of water channels, root hydraulic conductance and B absorption. Transgenic lines with variable expression of water channel proteins may be good test plants, with identical morphological and developmental characteristics.
On the other hand, chilling the canopy in the light impairs photosynthetic processes in leaf cells, through a range of ultrastructural, biochemical and molecular events (such as oxygen free-radical generation and anti-oxidation defence), which may eventually lead to irreversible photo-oxidative damage (Melis, 1999; Kratsch and Wise, 2000; Allen and Ort, 2001). These changes may be significantly modulated by B nutrition in leaf cells as B plays an important role in cell membrane integrity through forming B-complexes with membrane constituents containing cis-diol groups and in antioxidation systems in leaf cells (see review by Cakmak and Römheld, 1997). As a result, poor B nutrition in the shoot may lower chilling tolerance of leaf cells, exhibited as photo-oxidative damage in the light, particularly in chilling-sensitive crop species. Carefully designed experiments are required to test these hypotheses. Boron-buffered solution culture techniques (Huang et al., 1999) may be used to investigate B uptake kinetics in roots under specified chilling temperatures. To test if low B status increases the sensitivity of leaf cells to photo-inhibition, chilling responses of photosynthetic characteristics should be monitored at the on-set of chilling treatment over a time course, including chlorophyll fluorescence and the quantum yield of PS II photochemistry.
From a practical point of view, in chilling sensitive species, it is advisable to increase the B supply to the root zone in the cool season (such as early spring) as low root zone temperature is likely to lower B uptake capacity. When the occurrence of cold air currents is expected, preventive measures may be taken to boost shoot B status by foliar B application to minimize the risk of B deficiency-induced sensitivity to photoinhibition in leaf cells. This may also help to alleviate chilling damage in the young canopy of many tropical/subtropical woody species (e.g. teak, eucalyptus) in soils of low B status. In fact, B has been described as a ‘frost-protectant’ in field studies with horticultural and trees species, for example, in Prunus domestica (Hanson and Breen, 1985) and Eucalyptus grandis (Cooling, 1967). The effects of autumn B spray on flower development and fruit set in Prunus domestica was only evident in a cool spring (8 °C without any frost), when fruit set was increased by 32 % (Hanson and Breen, 1985). Field B fertilizer trials have provided direct evidence that increasing B supply to Eucalyptus grandis protected young trees from frost damage and shoot dieback in Zambia (Cooling and Jones, 1970).
Acknowledgments
Experimental work on B and low temperature in sunflower and oilseed rape was supported by the Australian Centre for International Agricultural Research. Professor Shu Fukai (School of Land and Food Sciences, The University of Queensland) helped revise the manuscript.
LITERATURE CITED
- Ahn SJ, Im YJ, Chung GC, Cho BH. 1999. Inducible expression of plasma membrane H+-ATPase in the roots of figleaf gourd plants under chilling root temperature. Physiologia Plantarum 106: 35–40. [Google Scholar]
- Allen DJ, Ort DR. 2001. Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends in Plant Science 6: 36–42. [DOI] [PubMed] [Google Scholar]
- Arato A, Bondarava N, Krieger-Liszkay A. 2004. Production of reactive oxygen species in chloride and calcium-depleted photosystem II and their involvement in photoinhibition. Biochimica et Biophysica Acta 1608: 171–180. [DOI] [PubMed] [Google Scholar]
- Aroca R, Tognoni F, Irigoyen JJ, Sanchez-Diaz M, Pardossi A. 2001. Different root low temperature response of two maize genotypes differing in chilling sensitivity. Plant Physiology and Biochemistry 39: 1067–1073. [Google Scholar]
- Aroca R, Irigoyen JJ, Sanchez-Diaz M. 2003. Drought enhances maize chilling tolerance. II. Photosynthetic traits and protective mechanisms against oxidative stress. Physiologia Plantarum 117: 540–549. [DOI] [PubMed] [Google Scholar]
- Aroca R, Vernieri P, Irigoyen JJ, Sanchez-Diaz M, Tognoni F, Pardossi A. 2003. Involvement of abscisic acid in leaf and root of maize (Zea mays L.) in avoiding chilling-induced water stress. Plant Science 165: 671–679. [Google Scholar]
- Asad A, Bell RW, Dell B. 2001. A critical comparison of the external and internal boron requirements for contrasting species in boron-buffered solution culture. Plant and Soil 233: 31–45. [Google Scholar]
- Barr R, Böttger M, Crane FL. 1993. The effect of boron on plasma membrane electron transport and associated proton secretion by cultured carrot cells. Biochemistry and Molecular Biology International 31: 31–39. [PubMed] [Google Scholar]
- Bell RW. 1997. Diagnosis and prediction of boron deficiency for plant production. Plant and Soil 193: 149–168. [Google Scholar]
- Blevins DG, Lukaszewski KM. 1998. Boron in plant structure and function. Annual Review in Plant Physiology and Plant Molecular Biology 49: 481–500. [DOI] [PubMed] [Google Scholar]
- Bloom AJ, Randall LB, Meyerhof PA, St Clair DA. 1998. The chilling sensitivity of root ammonium influx in a cultivated and wild tomato. Plant, Cell and Environment 21: 191–199. [Google Scholar]
- Bloom AJ, Zwieniecki MA, Passioura JB, Randall LB, Holbrook NM, St Clair DA. 2004. Water relations under root chilling in a sensitive and tolerant tomato species. Plant, Cell and Environment 27: 971–979. [Google Scholar]
- Bolwell GP, Butt VS. 1983. Photoinduced changes in o-diphenol oxidase and p-coumarate hydroxylase activities in spinach beet seedlings and leaves. Phytochemistry 22: 37–45. [Google Scholar]
- Brown PH, Shelp B. 1997. Boron mobility in plants. Plant and Soil 193: 85–102. [Google Scholar]
- Brown PH, Bellaloui N, Sah RN, Bassil E, Hu H. 2002. Uptake and transport of boron. In: Goldbach HE, Rerkasem B, Wimmer M, Brown PH, Thellier M, Bell RW, eds, Boron in plant and animal nutrition. New York: Kluwer Academic, 87–102. [Google Scholar]
- Brown PH, Bellaloui N, Wimmer MA, Bassil ES, Ruiz J, et al. 2002. Boron in plant biology. Plant Biology 4: 205–223. [Google Scholar]
- Browse J, Xin Z. 2001. Temperature sensing and cold acclimation. Current Opinion in Plant Biology 4: 241–246. [DOI] [PubMed] [Google Scholar]
- Cakmak I, Römheld V. 1997. Boron deficiency-induced impairments of cellular functions in plants. Plant and Soil 193: 71–84. [Google Scholar]
- Capell B, Dörffling K. 1993. Genotype-specific differences in chilling tolerance of maize in relation to chilling-induced changes in water status and abscisic acid accumulation. Physiologia Plantarum 88: 638–646. [DOI] [PubMed] [Google Scholar]
- Cara FA, Sanchez E, Ruiz JM, Romero L. 2002. Is phenol oxidation responsible for the short-term effects of boron deficiency on plasma-membrane permeability and function in squash roots? Plant Physiology and Biochemistry 40: 853–858. [Google Scholar]
- Carvajal M, Cooke DT, Clarkson DT. 1996. Responses of wheat plants to nutrient deprivation may involve the regulation of water channel function. Planta 199: 372–381. [Google Scholar]
- Chattopadhyay S, Datta SK, Mahato SB. 1994. Production of L-DOPA from cell suspension culture of Mucuna pruriens f. pruriens Plant Cell Report 13: 519–522. [DOI] [PubMed] [Google Scholar]
- Cleland RE. 1988. Molecular events of photoinhibitory inactivation in the reaction centre of photosystem II. Australian Journal of Plant Physiology 15: 135–150. [Google Scholar]
- Cooling EN. 1967. Frost resistance in Eucalyptus grandis following the application of fertilizer borate. Rhodesia, Zambia and Malawi Journal of Agricultural Research 5: 97–100. [Google Scholar]
- Cooling EN, Jones BE. 1970. The importance of boron and NPK fertilisers to eucalypts in the southern Province, Zambia. East African Agricultural and Forestry Journal 36: 185–194. [Google Scholar]
- Dannel F, Pfeffer H, Römheld V. 2000. Characterization of root boron pools, boron uptake and boron translocation in sunflower using the stable isotopes 10B and 11B. Australian Journal of Plant Physiology 27: 397–405. [Google Scholar]
- Dannel F, Pfeffer H, Römheld V. 2002. Update on boron in higher plants—uptake, primary translocation and compartmentation. Plant Biology 4: 193–204. [Google Scholar]
- Dell B, Malajczuk N. 1994. Boron deficiency in eucalypt plantations in China. Canadian Journal of Forest Research 24: 2409–2416. [Google Scholar]
- Dordas C, Brown PH. 2000. Permeability of boric acid across lipid bilayers and factors affecting it. Journal of Membrane Biology 175: 95–105. [DOI] [PubMed] [Google Scholar]
- Dordas C, Chrispeels MJ, Brown PH. 2000. Permeability and channel-mediated transport of boric acid across membrane vesicles isolated from squash roots. Plant Physiology 124: 1349–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shintinawy F. 1999. Structural and functional damage caused by boron deficiency in sunflower leaves. Photosynthetica 36: 565–573. [Google Scholar]
- Faris JA. 1926. Cold chlorosis of sugar cane. Phytopathology 16: 885–891. [Google Scholar]
- Forno DA, Asher CJ, Edwards DG. 1979. Boron nutrition of cassava, and the boron×temperature interaction. Field Crop Research 2: 265–279. [Google Scholar]
- Frommer WB, von Wiren N. 2002. Ping-pong with boron. Nature 410: 282–283. [DOI] [PubMed] [Google Scholar]
- Goldbach HE, Blaser-Grill J, Lindemann N, Porzelt M, Hörrmann C, Lupp B, et al. 1991. Influence of boron on net proton release and its relation to other metabolic processes. In: Randall DD, Blevins DG, eds, Current topics in plant biochemistry and physiology, Vol. 10. University of Missouri, Missouri, USA, 195–220. [Google Scholar]
- Goldbach HE, Wimmer MA, Chaumont F, Matoh T, Volkmann D, Baluška F, et al. 2002. Rapid responses of plants to boron deprivation. In: Goldbach HE, Rerkasem B, Wimmer M, Brown PH, Thellier M, Bell RW, eds. Boron in plant and animal nutrition. New York: Kluwer Academic, 167–180. [Google Scholar]
- Graham D, Patterson BD. 1982. Responses of plants to low, non-freezing temperatures: proteins, metabolism, and acclimation. Annual Review in Plant Physiology 33: 347–372. [Google Scholar]
- Hanson EJ, Breen PJ. 1985. Effects of all boron sprays and environmental factors on fruit set and boron accumulation in ‘Italian’ prune flowers. Journal of American Society and Horticulture Science 110: 389–392. [Google Scholar]
- Henzler T, Ye Q, Steudle E. 2004. Oxidative gating of water channels (aquaporins) in Chara by hydroxyl radicals. Plant, Cell and Environment 27: 1184–1195. [DOI] [PubMed] [Google Scholar]
- Hodges DM, Andrews CJ, Johnson DA, Hamilton RI. 1996. Antioxidant compound responses to chilling stress in differentially sensitive inbred maize lines. Physiologia Plantarum 98: 685–692. [Google Scholar]
- Hu H, Brown PH. 1997. Absorption of boron by plant roots. Plant and Soil 193: 49–58. [Google Scholar]
- Hu H, Brown PH, Labavitch JM. 1996. Species variability in boron requirement is correlated with cell wall pectin. Journal of Experimental Botany 47: 227–232. [Google Scholar]
- Huang L, Pant J, Bell RW, Dell B, Deane K. 1996. Effects of boron deficiency and low temperature on wheat sterility. In: Rawson HM and Subedi KD, eds. Sterility in wheat in subtropical Asia: extent, causes and solutions—Proceedings of a workshop 18–21 September 1995, Lumle Agricultural Research Centre, Pokhara, Nepal. Canberra: Australian Centre for International Agricultural Research, 90–101. [Google Scholar]
- Huang L, Bell RW, Dell B. 1999. Factors controlling equilibrium boron concentration in a B-buffered solution culture. Plant and Soil 208: 233–241. [Google Scholar]
- Huang L, Bell RW, Dell B. 2001. Boron supply into wheat (Triticum aestivum L. cv. Wilgoyne) ears whilst still enclosed within leaf sheath. Journal of Experimental Botany 52: 1731–1738. [PubMed] [Google Scholar]
- Huang L, Gherardi M, Bell RW, Dell B. 2002. High photon flux density increases external boron (B) requirements for leaf growth of sunflower (Helianthus annuus L. cv. Hysun 25) in B-buffered solution culture. In: Goldbach HE, Rerkasem B, Wimmer M, Brown PH, Thellier M, Bell RW, eds. Boron in plant and animal nutrition. New York: Kluwer Academic, 213–225. [Google Scholar]
- Hugly S, McCourt P, Browse J, Patterson GW, Somerville CR. 1990. A chilling sensitive mutant of Arabidopsis with altered sterol ester metabolism. Plant Physiology 93: 1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacperska A, Szaniawski RK. 1993. Frost resistance and water status of winter rape leaves as affected by differential shoot/root temperature. Physiologia Plantarum 89: 775–782. [Google Scholar]
- Kaldenhoff R, Grote K, Zhu J, Zimmerman U. 1998. Significance of plasmalemma aquaporins for water-transport in Arabdopsis thaliana The Plant Journal 14: 121–128. [DOI] [PubMed] [Google Scholar]
- Kang H-M, Saltveit ME. 2002. Effect of chilling on antioxidant enzymes and DPPH-radical scavenging activity of high- and low vigour cucumber seedling radicles. Plant, Cell and Environment 25: 1233–1238. [DOI] [PubMed] [Google Scholar]
- Kastori R, Plesnicar M, Pankovic D, Sakac Z. 1995. Photosynthesis, chlorophyll fluorescence and soluble carbohydrates in sunflower leaves as affected by boron deficiency. Journal of Plant Nutrition 18: 1751–1763. [Google Scholar]
- Kobayashi M, Mutoh T, Matoh T. 2004. Boron nutrition of cultured tobacco BY-2 cells. IV. Genes induced under low boron supply. Journal of Experimental Botany 55: 1441–1443. [DOI] [PubMed] [Google Scholar]
- Kratsch HA, Wise RR. 2000. The ultrastructure of chilling stress. Plant, Cell and Environment 23: 337–350. [Google Scholar]
- Kubacka-Zebalska M, Kacperska A. 1999. Low temperature-induced modifications of cell wall content and polysaccharide composition in leaves of winter oilseed rape (Brassica napus L., var. oleifera L.). Plant Science 148: 59–67. [Google Scholar]
- Li L, Li S, Tao Y, Kitagawa Y. 2000. Molecular cloning of a novel water channel from rice: its products expression in Xenopus oocytes and involvement in chilling tolerance. Plant Science 154: 43–51. [DOI] [PubMed] [Google Scholar]
- Lu C, Huang B. 2003. Effects of boron on membrane lipid peroxidation and endogenous protective systems in leaves of Eucalyptus grandis× Eucalyptus urophylla under low temperature. Journal of Tropical and Subtropical Botany 11: 217–222. [Google Scholar]
- Lukaszewski KM, Blevins DG. 1996. Root growth inhibition in boron-deficient or aluminium-stressed squash may be a result of impaired ascorbate metabolism. Plant Physiology 112: 1135–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons JM, Graham D, Raison JK. 1979.Low temperature stress in crop plants: the role of the membrane. New York: Academic Press. [Google Scholar]
- Macduff JH, Jackson SB. 1991. Growth and preferences for ammonium or nitrate uptake by barley in relation to root temperature. Journal of Experimental Botany 42: 521–530. [Google Scholar]
- Macduff JH, Wild A. 1989. Interactions between root temperature and nitrogen deficiency influence preferential uptake of NH+ and NO3− by oilseed rape. Journal of Experimental Botany 40: 195–206. [Google Scholar]
- Macduff JH, Hopper MJ, Wild A. 1987. The effect of root temperature on growth and uptake of ammonium and nitrate by Brassica napus L. cv. Bien venu in flowing solution culture. Journal of Experimental Botany 38: 53–66. [Google Scholar]
- Marschner H. 1995.Mineral nutrition of higher plants, 2nd edn. London: Academic Press. [Google Scholar]
- Matoh T. 1997. Boron in plant cell walls. Plant and Soil 193: 59–70. [Google Scholar]
- Matzner S, Comstock J. 2001. The temperature dependence of shoot hydraulic resistance: implications for stomatal behaviour and hydraulic limitation. Plant, Cell and Environment 24: 1299–1307. [Google Scholar]
- Melis A. 1999. Photosystem-II damage and repair cycle in chloroplasts: what modulates the rate of photodamage in vivo? Trends in Plant Science 4: 130–135. [DOI] [PubMed] [Google Scholar]
- Mole S, Ross JAM, Waterman PG. 1988. Light-induced variation in phenolics levels in foliage of rain-forest plants. I. Chemical changes. Journal of Chemical Ecology 14: 1–21. [DOI] [PubMed] [Google Scholar]
- Murata N, Nishiyama Y. 1998. Molecular mechanisms of the low-temperature tolerance of the photosynthetic machinery. In: Satoh K, Murata N, eds. Stress responses of photosynthetic organisms. Amsterdam: Elsevier Science. [Google Scholar]
- Park M, Li Q, Shcheynikov N, Zeng W, Muallem S. 2004. NaBC1 is a ubiquitous electrogenic Na+-coupled borate transporter essential for cellular boron homeostasis and cell growth and proliferation. Molecular Cell 16: 331–341. [DOI] [PubMed] [Google Scholar]
- Pettersson S. 1995. Low root zone temperature effects on net mineral nutrient uptake and distribution in barley (Hordeum vulgare). Journal of Plant Physiology 145: 459–464. [Google Scholar]
- Pfeffer H, Dannel F, Römheld V. 1998. Are there connections between phenol metabolism, ascorbate metabolism and membrane integrity in leaves of boron-deficient sunflower plants? Physiologia Plantarum 104: 479–485. [Google Scholar]
- Plesnicar M, Kastori R, Sakac Z, Pankovic D, Petrovic N. 1997. Boron as limiting factor in photosynthesis and growth of sunflower plants in relation to phosphate supply. Agrochimica 41: 144–154. [Google Scholar]
- Powles SB, Critchley C. 1980. The effect of photon flux density during growth on photo-inhibition of intact attached bean leaflets. Plant Physiology 65: 1181–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad TK, Anderson MD, Martin BA, Stewart CR. 1994. Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell 6: 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queiroz CGS, Alonso A, Mares-Guia M, Magalhaes AC. 1998. Chilling-induced changes in membrane fluidity and antioxidant enzyme activities in Coffea arabica L. roots. Biologia Plantarum 41: 403–413. [Google Scholar]
- Ruiz JM, Bretones G, Baghour M, Ragala L, Belakbir A, Romero L. 1998. Relationship between boron and phenolic metabolism in tobacco leaves. Phytochemistry 48: 269–272. [Google Scholar]
- Saruyama H, Tanida M. 1995. Effect of chilling on activated oxygen-scavenging enzymes in low temperature-sensitive and tolerant cultivars of rice (Oryza sativa L.). Plant Science 109: 105–113. [Google Scholar]
- Schon MK, Novacky A, Blevins DG. 1990. Boron induces hyperpolarisation of sunflower root cell membranes and increases membrane permeability to K+ Plant Physiology 93: 566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S, Shabala L. 2002. Kinetics of net H+, Ca2+, K+, Na+, NH4+, and Cl− fluxes associated with post-chilling recovery of plasma membrane transporters in Zea mays leaf and root tissues. Physiologia Plantarum 114: 47–56. [DOI] [PubMed] [Google Scholar]
- Shabala SN, Newman IA. 1997. H+ flux kinetics around plant roots after short-term exposure to low temperature: identifying critical temperatures for plant chilling tolerance. Plant, Cell and Environment 20: 1401–1410. [Google Scholar]
- Shorrocks VM. 1997. The occurrence and correction of boron deficiency. Plant and Soil 193: 121–148. [Google Scholar]
- Smart DR, Bloom AJ. 1991. Influence of root NH4+ and NO3− content on the temperature response of net NH4+ and NO3− uptake in chilling sensitive and chilling resistant Lycopersicon taxa. Journal of Experimental Botany 42: 331–338. [Google Scholar]
- Sonoike K. 1999. The different roles of chilling temperatures in the photoinhibition of photosystem I and photosystem II. Journal of Photochemistry and Photobiology B: Biology 48: 136–141. [Google Scholar]
- Steudle E. 2000. Water uptake by roots: effects of water deficit. Journal of Experimental Botany 51: 1531–1542. [DOI] [PubMed] [Google Scholar]
- Steudle E, Peterson CA. 1998. How does water get through roots? Journal of Experimental Botany 49: 775–788. [Google Scholar]
- Szaniawski RK, Kielkiewicz M. 1982. Maintenance and growth respiration in shoots and roots of sunflower plants grown at different root temperatures. Physiologia Plantarum 54: 500–504. [Google Scholar]
- Takano J, Nguchi K, Yasumori M, Kobayashi M, Gajdos Z, Miwa K, et al. 2002.Arabidopsis boron transporter for xylem loading. Nature 420: 337–340. [DOI] [PubMed] [Google Scholar]
- Terashima I, Noguchi K, Itoh-Nemoto T, Park Y-M, Kubo A, Tanaka K. 1998. The cause of PSI photoinhibition at low temperatures in leaves of Cucumis sativus, a chilling-sensitive plant. Physiologia Plantarum 103: 295–303. [Google Scholar]
- Tindall JA, Mills HA, Radcliffe DE. 1990. The effect of root zone temperature on nutrient uptake of tomato. Journal of Plant Nutrition 13: 939–956. [Google Scholar]
- Tyerman SD, Bohnert HJ, Maurel C, Steudle E, Smith JA. 1999. Plant aquaporins: their molecular biology, biophysics and significance for plant water relations. Journal of Experimental Botany 25: 1055–1071. [Google Scholar]
- Tyerman SD, Niemietz CM, Bramley H. 2002. Plant aquaporins: multifunctional water and solute channels with expanding roles. Plant, Cell and Environment 25: 173–194. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Tang YL, Zhang FS, Wang H. 1999. Effect of boron and low temperature on membrane integrity of cucumber leaves. Journal of Plant Nutrition 22: 543–550. [Google Scholar]
- Wise RR, McWilliam J, Naylor AW. 1983. A comparative study of low temperature-induced ultrastructure alterations of three species with different chilling sensitivities. Plant, Cell and Environment 6: 525–535. [Google Scholar]
- Ye Z. 2005.Effect of low temperature on plant boron nutrition. PhD Thesis, Murdoch University. [Google Scholar]
- Ye Z, Bell RW, Dell B, Huang L. 2000. Response of sunflower to boron supply at low root zone temperature. Communication in Soil Science and Plant Analysis 31: 2379–2392. [Google Scholar]
- Ye Z, Huang L, Bell RW, Dell B. 2003. Low root zone temperature favours shoot B partitioning into young leaves of oilseed rape (Brassica napus). Physiologia Plantarum 118: 213–220. [Google Scholar]
- Yu Ch-W, Murphy TM, Sung W-W, Lin Ch-H. 2002. H2O2 treatment induces glutathione accumulation and chilling tolerance in mung bean. Functional Plant Biology 29: 1081–1087. [DOI] [PubMed] [Google Scholar]