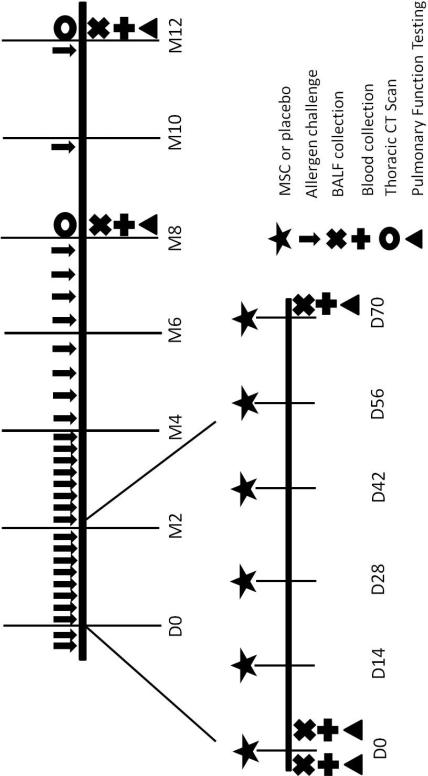
Figure 1. Schematic Overview of Study Design.
After induction of asthma and chronic allergen challenge for 9 months, cats were enrolled in the study and randomized to receive 6 bimonthly infusions of MSC (n=5) or PBS (n=4). During the remaining study period, no further experimental treatments were administered. Allergen challenges were performed weekly for 4 months after the first infusions. Subsequent challenges were performed bimonthly between months 4 and 8 and monthly from 8 months until study end. Blood and BALF were collected and pulmonary function testing was performed at baseline and days 3, 75, 240, and 367. Thoracic CT scans were acquired at month 8 and 12 after the first experimental infusion.