Abstract
Background
The aim of this study was to determine if ultrasound (US) measurements of anterior neck soft tissue thickness at hyoid bone (DSHB), thyrohyoid membrane (DSEM), and anterior commissure (DSAC) levels can be used to predict difficult laryngoscopy.
Material/Methods
We included 203 patients age 20–65 years scheduled to undergo general anesthesia in this prospective observational study. Correlation analysis and receiver operating characteristic curve (ROC) analysis were used to determine the roles of screening tests [interincisor gap (IIG), thyromental distance (TMD), modified Mallampati score (MMS)] and US measurements (DSHB, DSEM, DSAC) in predicting difficult laryngoscopy.
Results
There were 28 out of 203 patients categorized as difficult laryngoscopy. DSHB, DSEM, DSAC, and MMS were greater in the difficult laryngoscopy group (P<0.0001). There was a strong positive correlation between DSEM and DSHB (r=0.74); moderate positive correlations between DSEM and DSAC (r=0.60), DSHB and DSAC (r=0.69); small positive correlations between MMS and DSHB (r=0.32), MMS and DSEM (r=0.27), MMS and DSAC (r=0.32), all P values ≤0.0001; very small positive correlation between TMD and IIG (r=0.18, P=0.0089); small negative correlation between IIG and MMS (r=−0.27, P=0.0001); and very small negative correlations between MMS and TMD (r=−0.20, P=0.004), IIG and DSAC (r=−0.18, P=0.011), IIG and DSHB (r=−0.15, P=0.034). The areas under the ROC curve (AUCs) of MMS, DSHB, DSEM, and DSAC were significantly larger compared with the reference line (P<0.0001).
Conclusions
Anterior neck soft tissue thicknesses measured by US at hyoid bone, thyrohyoid membrane, and anterior commissure levels are independent predictors of difficult laryngoscopy. Combinations of those screening tests or risk factors with US measurements might increase the ability to predict difficult laryngoscopy.
MeSH Keywords: Airway Management, Anesthesia, Critical Care, Laryngoscopy, Ultrasonography
Background
Endotracheal intubation is one of the most important skills for anesthesiologists in securing the airway during general anesthesia and resuscitation. Failure to secure the airway can cause anesthesia-related life-threatening morbidity and mortality. Therefore, unanticipated difficult intubation remains a primary concern for anesthesiologists [1]. Theoretically, accurate preoperative airway evaluation can reduce or avoid unanticipated difficult intubation. However, the difficult laryngoscopy and tracheal intubation rate still remains at 1.5–13% due to poor reliability of traditional protocols, algorithms, and combinations of screening tools in identifying a potentially difficult airway [2].
Due to the portable, noninvasive characteristics, point-of-care ultrasound (US) technique has been widely used in the operating room for ultrasound-guided nerve block, central venous access, and pneumothorax diagnosis. With improved visualization of airway structures, more studies have been focusing on airway structure and function [3,4]. Prasad et al. first found that US can reliably image all of the structures visualized by CT, and the infrahyoid airway structure parameters measured by ultrasound agree well with the parameters measured by CT [3]. Adhikari et al. further demonstrated that the anterior neck soft tissue thickness measured by ultrasound at hyoid bone and thyrohyoid membrane levels can be used as an index to predict difficult laryngoscopy, but only the anterior neck soft tissue thickness at thyrohyoid membrane levels can be used as an independent predictor of difficult laryngoscopy. Interestingly, they did not find a correlation between US measurements and clinical screening tests [5]. Ezri et al. [6] also found that an abundance of fat tissue at the anterior neck region as detected by ultrasound in Israeli obesity patients was a very good independent predictor of difficult laryngoscopy, being a much better predictor than body mass index (BMI) per se. However, a similar study in the Unites States showed that US quantification of anterior soft tissue and general bedside screening tests failed to predict difficult laryngoscopy in American obese patients [7], suggesting that racial or body shape differences might exist.
To define the role of airway US in predicting difficult laryngoscopy, we evaluated the feasibility of ultrasound in predicting difficult laryngoscopy in a Chinese Han population.
Material and Methods
This was a prospective observational study. After approval of the research protocol by the local hospital ethics committee for human studies and obtaining personal informed consent, 203 American Society of Anesthesiologists (ASA) Grade I and II adult patients undergoing elective surgeries and receiving general anesthesia were included in this study. Exclusion criteria included patients who had facial, cervical, pharyngeal and epiglottis surgical or trauma history, patients with most teeth lost, and patients with arthritis.
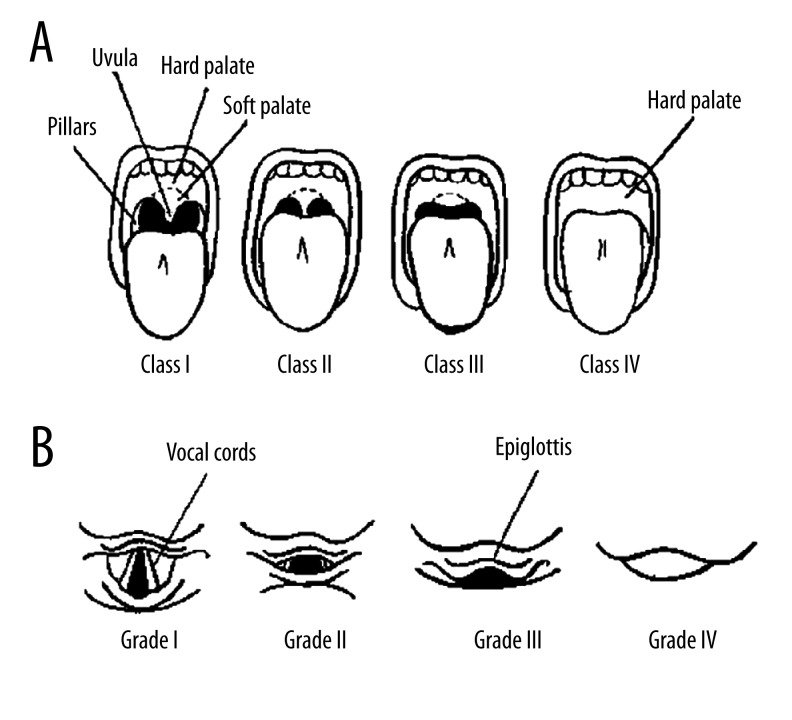
The modified Mallampati score (MMS) was specified according to the visibility of pharyngeal structures with the patient in an upright sitting position, head in neutral position, mouth wide open, and tongue protruding to its maximum without phonation (Figure 1A) [8]. Class I is visualization of the hard palate, soft palate, fauces, uvula, and pillars. Class II is visualization of the hard palate, soft palate, fauces, and base of uvula. Class III is visualization of the hard palate and soft palate. Class IV is visualization of only the hard palate.
Figure 1.
Modified Mallampati scoring (A) and Cormack-Lehane grading of glottis exposure on direct laryngoscopic views (B). (A) Class I: visualization of the hard palate, soft palate, fauces, uvula and pillars; Class II: visualization of the hard palate, soft palate, fauces, and base of uvula; Class III: visualization of the hard palate, soft palate; Class IV: only hard palate is visible. (B) Grade I: full view of the glottis; Grade II: partial view of the glottis or arytenoids; Grade III: only the epiglottis is seen; and Grade IV: neither glottis nor epiglottis are visible. Grade I and II were categorized as easy laryngoscopy; Grade III and IV were categorized as difficult laryngoscopy.
Thyromental distance (TMD) was measured from the mental prominence to the thyroid cartilage with the patient’s neck extended fully.
Interincisor gap (IIG) was measured from the upper central incisors to the lower central incisors while the patient’s mouth was fully opened.
Ultrasound measurements were performed by the primary investigator with the patient supine and the head and neck in neutral position. The thicknesses of anterior neck soft tissue at hyoid bone, thyrohyoid membrane, and anterior commissure levels were obtained transversely across the anterior surface of the neck with a 13–6 MHz HFL38× linear array ultrasound probe attached to a SonoSite S-nerve machine (SonoSite Inc., Bothell, WA, USA). At hyoid bone level, the minimal distance from the hyoid bone to skin surface (DSHB) was measured (Figure 2A, 2B). At thyrohyoid membrane level, the distance from skin to epiglottis midway (DSEM) between the hyoid bone and thyroid cartilage was measured (Figure 2C, 2D). At anterior commissure level, the minimal distance from skin to anterior commissure (DSAC) was obtained (Figure 2E, 2F).
Figure 2.
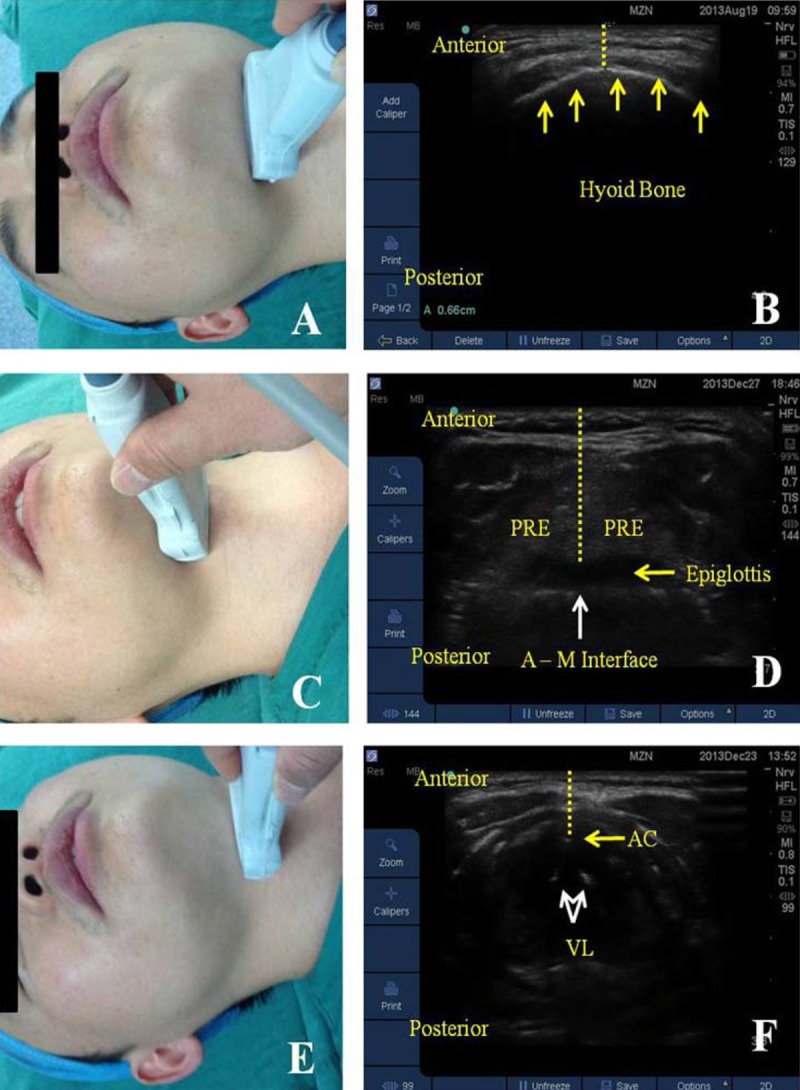
Ultrasound measurements of anterior neck soft tissue thicknesses. Left panel: different neck levels with ultrasound probe in transverse position. (A) hyoid bone level; (C) thyrohyoid membrane level; (E) anterior commissure level; Right panel: corresponding ultrasound images. (B) yellow arrows denote hyoid bone, yellow dotted line denotes the minimum distance from skin to hyoid bone (DSHB); (D) yellow dotted line denotes the distance from skin to epiglottis midway (DSEM) between the hyoid bone and thyroid cartilage, PRE (pre-epiglottic space), A–M interface (air-mucosal); (F) yellow arrows denote anterior commissure, yellow dotted line denotes the minimum distance from skin to anterior commissure (DSAC), VL (vocal cord, white arrows).
After anesthesia induction with midazolam 0.04 mg/kg, propofol 2–2.5 mg/kg, fentanyl 2–4 μg/kg, and succinylcholine 2 mg/kg, endotracheal intubation was carried out by anesthesia providers with a minimum of 2 years of endotracheal intubation experience. All the patients were in neutral position without neck overextension or over-bending. The Macintosh blades were used to expose the target larynx, and no external laryngeal pressure was used to facilitate this process. Classification of laryngoscopic views was based on the method described by Cormack and Lehane (Figure 1B) [9]. Grade I is full view of the glottis. Grade II is partial view of the glottis or arytenoids. Grade III is only epiglottis seen. Grade IV is neither glottis nor epiglottis visible. Grade I and II are categorized as easy laryngoscopy. Grade III or IV are categorized as difficult laryngoscopy.
Statistical analysis
Continuous data are expressed as means (standard deviation [SD]); categorical data are expressed as numbers of occurrences (percents). Laryngoscopy graded III or IV were defined as difficult. Easy and difficulty laryngoscopies were compared. Comparison analysis was performed using the t-test for continuous variables and chi-square or Fisher exact tests, as appropriate, for non-continuous variables. Correlation analysis was performed using the Pearson test. The best fit lines of DSHB, DSEM, DSAC, MMS, IIG, and TMD were determined using a least-squares regression technique. Receiver operating characteristic (ROC) analyses were used to calculate the comparable threshold values of DSHB, DSEM, DSAC, TMD, IIG, and MMS. The areas under the curves (AUCs) were compared using the DeLong method [10]. Optimal cutoff values were calculated using the Youden index (calculated as: sensitivity + specificity − 100). Statistical analyses were performed using MedCalc for Windows, version 12.6.1.0 (MedCalc Software, Ostend, Belgium). The level of statistical significance was P<0.05, and P<0.0001 was considered to be very statistically significant.
Results
A total of 203 eligible patients (120 females, 83 males) were included in this study. The demographic characteristics are shown in Table 1. There were 28 of 203 patients (13.8%) categorized as difficult laryngoscopy, and 50% of patients with difficult laryngoscopy in our study were males. No differences were noted in sex, age, and height, but BW and BMI values in the difficult laryngoscopy group were higher. The BMI value was 25.63±2.80 kg/m2 for the difficult laryngoscopy group, and 23.61±3.43 kg/m2 for the easy laryngoscopy group (P<0.05).
Table 1.
Basic characteristics of the patients with difficult and easy laryngoscopy.
| Parameters | Difficult (n=28) | Easy (n=175) | P-value |
|---|---|---|---|
| Gender (M/F) | 14 (50%)/14(50%) | 69(39.4%)/106(60.6%) | 0.396 |
| Age (yr) | 46±15 | 47±14 | 0.984 |
| Weight (kg) | 69.30±9.25 | 63.52±10.28 | 0.0056 |
| Height (cm) | 164.39±8.35 | 163.98±7.21 | 0.785 |
| BMI (kg/m2) | 25.63±2.80 | 23.61±3.43 | 0.0034 |
Data are presented as mean ±SD or number of patients (percent).
Airway evaluation parameters, including MMS, TMD, IIG, DSHB, DSEM, and DSAC, are shown in Table 2. The thicknesses of anterior neck soft tissue measured by US were greater in the difficult laryngoscopy group compared to the easy laryngoscopy group at the level of the hyoid bone, thyrohyoid membrane, and anterior commissure. MMS in the difficult laryngoscopy group was higher than in the easy group. However, no statistically significant difference was found between easy and difficult laryngoscopy groups for TMD and IIG.
Table 2.
Airway evaluating parameters for predicting difficult laryngoscopy.
| Parameters | Difficult (N=28) | Easy (N=175) | P-value |
|---|---|---|---|
| MMS | <0.0001 | ||
| I | 1 (3.6%) | 42 (24.0%) | |
| II | 13 (46.4%) | 102 (58.3%) | |
| III | 13 (46.4%) | 31 (17.7%) | |
| IV | 1 (3.6%) | 0 (0.0%) | |
| TMD | 6.71±0.94 | 6.77±0.70 | 0.664 |
| IIG | 3.74±0.43 | 3.86±0.47 | 0.211 |
| DSHB | 1.51±0.27 | 0.98±0.26 | <0.0001 |
| DSEM | 2.39±0.34 | 1.49±0.39 | <0.0001 |
| DSAC | 1.30±0.31 | 0.92±0.20 | <0.0001 |
Data are presented as mean ±SD or number of patients (percent). MMS – Modified Mallampati Score; TMD – thyromental distance; IIG – inter incisor gap; DSHB – distance between skin and hyoid bone; DSEM – distance between skin and epiglottis; DSAC – distance between skin and anterior commissure.
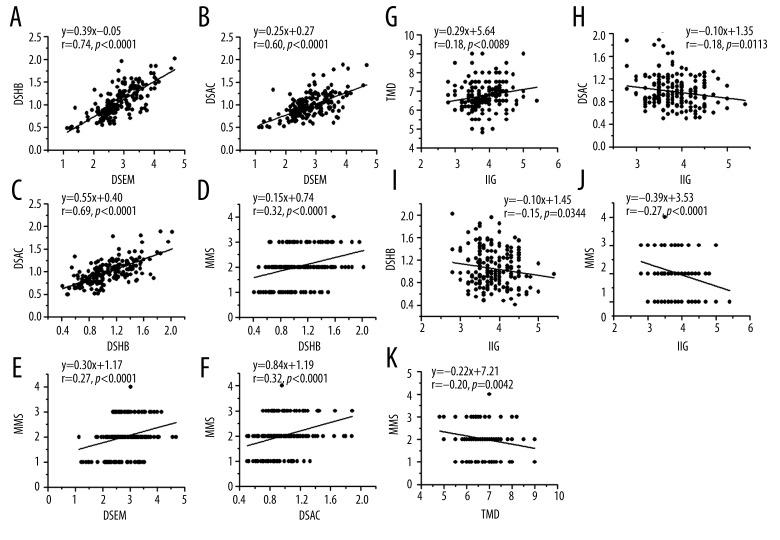
A strong positive correlation existed between DSEM and DSHB; moderate positive correlations between DSEM and DSAC, DSHB and DSAC; small positive correlations between MMS and DSHB, MMS and DSEM, MMS and DSAC; and very small positive correlations between TMD and IIG. The correlation coefficients along with 95% confidence interval (CI) between DSEM and DSHB, DSEM and DSAC, DSHB and DSAC, MMS and DSHB, MMS and DSEM, MMS and DSAC, and between TMD and IIG were 0.74 (0.67–0.80, P<0.0001), 0.60 (0.50–0.68, P<0.0001), 0.69 (0.61–0.75, P<0.0001), 0.32 (0.19–0.44, P<0.0001), 0.27 (0.14–0.40, P=0.0001), 0.32 (0.19–0.44, P<0.0001), 0.18 (0.05 to 0.31, P=0.0089), respectively (Figure 3A–3G). Small negative correlation were found between IIG and MMS (r=−0.27, P=0.0001); and very small negative correlations were found between MMS and TMD (r=−0.20, P=0.0042), IIG and DSAC (r=−0.18, P=0.0113), and IIG and DSHB (r=−0.15, P=0.0344) (Figure 3H–3K). No correlations were found between TMD and DSEM, TMD and DSHB, and TMD and DSAC (data not shown).
Figure 3.
Relationships between the ultrasound measurements of anterior neck soft tissue thicknesses and the screening tests in predicting difficult laryngoscopy. The solid lines are regression lines. r=correlation coefficient. DSHB: the minimum distance from skin to hyoid bone; DSEM: the distance from skin to epiglottis midway between the hyoid bone and thyroid cartilage; DSAC: the minimum distance from skin to anterior commissure; IIG: Interincisor gap; MMS: modified Mallampati score.
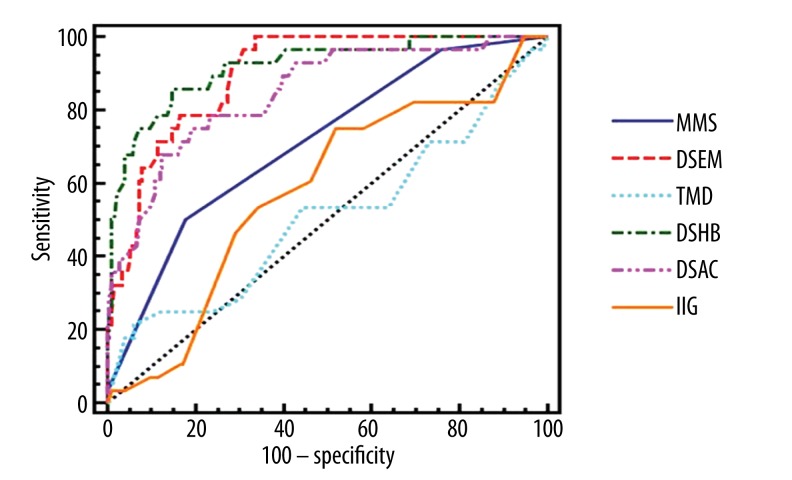
To further assess the roles of MMS, IIG, TMD, DSHB, DSEM, and DSAC in predicting difficult laryngoscopy, the ROC curves (Figure 4) were drawn using MedCalc software with Laryngoscopy grade over II as the threshold of difficult laryngoscopy [5]. As determined by the Youden index, the optimal cutoff values (with sensitivity and specificity in parentheses) for MMS, IIG, TMD, DSHB, DSEM, and DSAC to predict difficult laryngoscopy were over 2 (50.0%, 82.3%), 3.8 cm (75.0%, 48.0%), 5.9 cm (21.4%, 93.7%), 1.28 cm (85.7%, 85.1%), 1.78 cm (100.0%, 66.3%), and 1.1 cm (75.0%, 80.6%), respectively. The areas under the ROC curve (AUCs) were significantly different from the area under the reference line (area=0.5) for MMS, DSHB, DSEM, and DSAC; and the P values of MMS, DSHB, DSEM, and DSAC were all less than 0.0001. No difference between the AUCs of TMD, IIG, and the area under the reference line were identified, suggesting that MMS, DSHB, DSEM, and DSAC might be better than TMD and IIG in predicting difficult laryngoscopy (Table 3).
Figure 4.
Receiver operating characteristic (ROC) analyses for DSHB (green dotted line), DSEM (red dotted line), DSAC (pink dotted line), MMS (solid blue line), TMD (cyan dotted line), and IIG (solid brown line). Cormack-Lehane grading of glottis exposure over II was considered the threshold of difficult laryngoscopy during the study. Black dotted line=reference line. DSHB: the minimum distance from skin to hyoid bone; DSEM: the distance from skin to epiglottis midway between the hyoid bone and thyroid cartilage; DSAC: the minimum distance from skin to anterior commissure; MMS: modified Mallampati score; TMD: thyromental distance; and IIG: interincisor gap.
Table 3.
The area under the ROC curves (AUC) for DSHB, DSEM, DSAC, MMS, IIG, and TMD.
| Parameters | AUC ±SE | 95% CI | P |
|---|---|---|---|
| DSHB | 0.92±0.03 | 0.87 to 0.95 | <0.0001 |
| DSEM | 0.90±0.03 | 0.85 to 0.94 | <0.0001 |
| DSAC | 0.85±0.04 | 0.79 to 0.89 | <0.0001 |
| MMS | 0.71±0.05 | 0.64 to 0.77 | <0.0001 |
| IIG | 0.58±0.06 | 0.51 to 0.65 | 0.17 |
| TMD | 0.51±0.07 | 0.44 to 0.58 | 0.89 |
AUC ±SE – area under the ROC curves ± Standard Error; 95% CI – 95% Confidence Interval; DSHB – distance between skin and hyoid bone; DSEM – distance between skin and epiglottis; DSAC – distance between skin and anterior commissure; MMS – Modified Mallampati Score; IIG – inter incisor gap; TMD – thyromental distance.
Discussion
With the rapid expansion of portable US in anesthesia for guiding the placement of central venous catheters and the performance of regional block [11–13], some preliminary results exploring the feasibility of portable US in airway evaluation and management have been reported, even though the air attenuates US transmission and thereby creates artifacts [3–5,14,15]. In the present study, we compared the role of the US measurements and traditional screening tests, including IIG, TMD, and MMS, in predicting difficult laryngoscopy in a Chinese Han population. We found that strong positive linear correlations existed among the thicknesses of anterior neck soft tissue measured by US at hyoid bone, thyrohyoid membrane, and anterior commissure levels. The AUCs of MMS, DSHB, DSEM, and DSAC are all over 0.7, indicating they are all good parameters in predicting difficult laryngoscopy. The AUCs of TMD and IIG were less than 0.7, suggesting that TMD and IIG were poor parameters in predicating difficult laryngoscopy.
To clearly visualize the glottis and smoothly finish the intubation procedure, anesthesiologists need to place the laryngoscope blade to the base of the epiglottis, and then lift it up. Therefore, in addition to the performer’s skills and experience, many factors, including dental status, mouth opening, oropharyngeal space, mandibular space, and neck motility, are all involved this complicated procedure [16,17]. Any of these factors can affect the recognition of difficult airway and preoperative evaluation. MMS mainly reflects the size of the tongue relative to the oral cavity, or the oropharyngeal space [18–20], IIG means the mouth opening, and TMD can reflect the length of the neck. The US measurements mainly reflect the thickness of anterior neck soft tissue.
The Mallapati score was first introduced by Mallampati et al. in 1985, and then modified by Samsoon and Young in 1987 [8,18]. Due to the simple and clear evaluation standards, MMS has become one of the most commonly used tools to predict a difficult airway. However, MMS mainly depends on patient cooperation and body position, which can greatly affect the accuracy of airway evaluation [21]. Therefore, MMS has been reported to be a good predictor by many, but was found to be of limited value by others [22]. A recent meta-analysis involving 177 088 patients demonstrate that MMS is inadequate as a stand-alone test to predict difficult laryngoscopy or tracheal intubation, but it may well be a part of a multifactorial model for the prediction of a difficult tracheal intubation [23]. Our results also confirmed this conclusion.
IIG has been repeatedly reported to be associated with difficult intubation [24,25], but our results showed that there was no significant difference in mean IIG between difficult and easy laryngoscopy groups (3.74±0.43 vs. 3.86±0.47 cm). Furthermore, the AUC of IIG was 0.58±0.06, which was far less than 0.7, suggesting that IIG might be not a good screening parameter for predicting difficult laryngoscopy in our study. Our IIG conclusion was similar to the previous report by Savva [26], but different from results of other studies [6,27]. Whether this difference was really caused by racial factors requires further investigation because the measurements of IIG need patients to fully cooperate. TMD is also used as a screening test to predict difficult airway, but our results suggest that TMD is not a useful screening test. However, we could not determine whether this was because of limited patient numbers or because TMD was not useful in predicting difficult airway due to the small sample size.
CT, MRI, and other imaging techniques can accurately measure the thickness of anterior neck soft tissue, but they are expensive and unavailable in many operating rooms. Portable US is inexpensive, rapid, and convenient to perform in the operating room, and most importantly, it can quantify the neck fat thickness as accurately as MI [28]. However, the results of 2 prior studies focusing on soft tissue thickness at and below the level of the vocal cords in a specific group of obese patients are inconsistent [6,7]. Adhikari et al. further measured the anterior neck soft tissue at the hyoid bone and thyrohyoid membrane levels, which is important to displace the glottis by the laryngoscopic blade, and found that US measurements of anterior neck soft tissue thickness at the level of hyoid bone and thyrohyoid membrane can be used to predict difficult laryngoscopies, and a 2.8-cm US measurement at the thyrohyoid membrane was a good independent predictor of difficult laryngoscopy [5]. Our results show that the thicknesses of anterior neck soft tissue at the level of the hyoid bone (1.51 [95% CI=1.40–1.61] cm vs. 0.98 [95% CI=0.94–1.02] cm), the thyrohyoid membrane (2.39 [95% CI=2.17–2.62] cm vs. 1.49 [95% CI=1.43–1.55] cm), and the anterior commissure (1.30 [95% CI=1.18–1.42] cm vs. 0.92 [95% CI=0.87–0.94] cm) were greater in the difficult laryngoscopy group and were significantly correlated. Furthermore, the ranges of anterior neck soft tissue for those with difficult laryngoscopy were mutually exclusive from those patients with an easy laryngoscopy, indicating that they are independent predictors of difficult laryngoscopy, even though the ranges of these 3 parameters were smaller than in a previous report [5].
Several limitations exist in our study. Glottis exposure by place laryngoscope is a very complicated procedure, and many subjective and objective factors such as the provider’s skills and experience, airway secretions, and abnormalities of anatomical structures are involved in this procedure. Therefore, the small sample size might limit our conclusions. The investigators were not totally blinded to the study purpose, and some clinical signs might indicate the possibility of difficult laryngoscopy, which can cause some bias during US measurements. Due to the low incidence of difficult laryngoscopy, it is impossible for us to randomly assign an equal number of patients to both groups, thus there were 28 patients in the difficult group and 175 patients in the easy group.
Conclusions
Anterior neck soft tissue thicknesses measured by US at the hyoid bone, thyrohyoid membrane, and anterior commissure levels are independent predictors of difficult laryngoscopy. The commonly used screening tests for difficult intubation have only poor-to-moderate predictive power when used alone. Combinations of these screening tests or risk factors with US measurements might increase the ability to predict difficult laryngoscopy.
Acknowledgments
Thanks to Long Liu M.D. and Qingsheng Shi M.D. (Department of Diagnostic Ultrasound, Shanghai Jiaotong University Affiliated Shanghai First People’s Hospital, Shanghai, China) for directing ultrasound measurements.
Footnotes
Disclosure of potential conflicts of interest
The authors state no potential conflicts of interest.
Source of support: This work was supported by the National Natural Science Foundation of China (81271263 to J.J.Z), Shanghai Pujiang Talent Program from Science and Technology Commission of Shanghai Municipality, China (11PJ1408000 to J.J.Z), and Medical Climbing Program from Songjiang Health Bureau, China (2011PD15 to J.J.Z)
References
- 1.Law JA, Broemling N, Cooper RM, et al. The difficult airway with recommendations for management – part 1 – difficult tracheal intubation encountered in an unconscious/induced patient. Can J Anaesth. 2013;60:1089–118. doi: 10.1007/s12630-013-0019-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan ZH, Kashfi A, Ebrahimkhani E. A comparison of the upper lip bite test (a simple new technique) with modified Mallampati classification in predicting difficulty in endotracheal intubation: a prospective blinded study. Anesth Analg. 2003;96:595–99. doi: 10.1097/00000539-200302000-00053. [DOI] [PubMed] [Google Scholar]
- 3.Prasad A, Yu E, Wong DT, et al. Comparison of sonography and computed tomography as imaging tools for assessment of airway structures. J Ultrasound Med. 2011;30:965–72. doi: 10.7863/jum.2011.30.7.965. [DOI] [PubMed] [Google Scholar]
- 4.Kristensen MS. Ultrasonography in the management of the airway. Acta Anaesthesiol Scand. 2011;55:1155–73. doi: 10.1111/j.1399-6576.2011.02518.x. [DOI] [PubMed] [Google Scholar]
- 5.Adhikari S, Zeger W, Schmier C, et al. Pilot study to determine the utility of point-of-care ultrasound in the assessment of difficult laryngoscopy. Acad Emerg Med. 2011;18:754–58. doi: 10.1111/j.1553-2712.2011.01099.x. [DOI] [PubMed] [Google Scholar]
- 6.Ezri T, Gewurtz G, Sessler DI, et al. Prediction of difficult laryngoscopy in obese patients by ultrasound quantification of anterior neck soft tissue. Anaesthesia. 2003;58:1111–14. doi: 10.1046/j.1365-2044.2003.03412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komatsu R, Sengupta P, Wadhwa A, et al. Ultrasound quantification of anterior soft tissue thickness fails to predict difficult laryngoscopy in obese patients. Anaesth Intensive Care. 2007;35:32–37. doi: 10.1177/0310057X0703500104. [DOI] [PubMed] [Google Scholar]
- 8.Samsoon GL, Young JR. Difficult tracheal intubation: a retrospective study. Anaesthesia. 1987;42:487–90. doi: 10.1111/j.1365-2044.1987.tb04039.x. [DOI] [PubMed] [Google Scholar]
- 9.Cormack RS, Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia. 1984;39:1105–11. [PubMed] [Google Scholar]
- 10.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 11.Marhofer P, Willschke H, Kettner S. Current concepts and future trends in ultrasound-guided regional anesthesia. Curr Opin Anaesthesiol. 2010;23:632–36. doi: 10.1097/ACO.0b013e32833e2891. [DOI] [PubMed] [Google Scholar]
- 12.Moore CL. Ultrasound first, second, and last for vascular access. J Ultrasound Med. 2014;33:1135–42. doi: 10.7863/ultra.33.7.1135. [DOI] [PubMed] [Google Scholar]
- 13.Hind D, Calvert N, McWilliams R, et al. Ultrasonic locating devices for central venous cannulation: meta-analysis. BMJ. 2003;327:361. doi: 10.1136/bmj.327.7411.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta D, Srirajakalidindi A, Ittiara B, et al. Ultrasonographic modification of Cormack Lehane classification for pre-anesthetic airway assessment. Middle East J Anesthesiol. 2012;21:835–42. [PubMed] [Google Scholar]
- 15.Hui CM, Tsui BC. Sublingual ultrasound as an assessment method for predicting difficult intubation: a pilot study. Anaesthesia. 2014;69:314–19. doi: 10.1111/anae.12598. [DOI] [PubMed] [Google Scholar]
- 16.Shiga T, Wajima Z, Inoue T, Sakamoto A. Predicting difficult intubation in apparently normal patients: a meta-analysis of bedside screening test performance. Anesthesiology. 2005;103:429–37. doi: 10.1097/00000542-200508000-00027. [DOI] [PubMed] [Google Scholar]
- 17.Langeron O, Cuvillon P, Ibanez-Esteve C, et al. Prediction of difficult tracheal intubation: time for a paradigm change. Anesthesiology. 2012;117:1223–33. doi: 10.1097/ALN.0b013e31827537cb. [DOI] [PubMed] [Google Scholar]
- 18.Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J. 1985;32:429–34. doi: 10.1007/BF03011357. [DOI] [PubMed] [Google Scholar]
- 19.Randell T. Prediction of difficult intubation. Acta Anaesthesiol Scand. 1996;40:1016–23. doi: 10.1111/j.1399-6576.1996.tb05620.x. [DOI] [PubMed] [Google Scholar]
- 20.Huang HH, Lee MS, Shih YL, et al. Modified Mallampati classification as a clinical predictor of peroral esophagogastroduodenoscopy tolerance. BMC Gastroenterol. 2011;11:12. doi: 10.1186/1471-230X-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calder I, Picard J, Chapman M, et al. Mouth opening: a new angle. Anesthesiology. 2003;99:799–801. doi: 10.1097/00000542-200310000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Lee A, Fan LT, Gin T, et al. A systematic review (meta-analysis) of the accuracy of the Mallampati tests to predict the difficult airway. Anesth Analg. 2006;102:1867–78. doi: 10.1213/01.ane.0000217211.12232.55. [DOI] [PubMed] [Google Scholar]
- 23.Lundstrom LH, Vester-Andersen M, Moller AM, et al. Poor prognostic value of the modified Mallampati score: a meta-analysis involving 177 088 patients. Br J Anaesth. 2011;107:659–67. doi: 10.1093/bja/aer292. [DOI] [PubMed] [Google Scholar]
- 24.Wilson ME, Spiegelhalter D, Robertson JA, Lesser P. Predicting difficult intubation. Br J Anaesth. 1988;61:211–16. doi: 10.1093/bja/61.2.211. [DOI] [PubMed] [Google Scholar]
- 25.Karkouti K, Rose DK, Wigglesworth D, Cohen MM. Predicting difficult intubation: a multivariable analysis. Can J Anaesth. 2000;47:730–39. doi: 10.1007/BF03019474. [DOI] [PubMed] [Google Scholar]
- 26.Savva D. Prediction of difficult tracheal intubation. Br J Anaesth. 1994;73:149–53. doi: 10.1093/bja/73.2.149. [DOI] [PubMed] [Google Scholar]
- 27.El-Ganzouri AR, McCarthy RJ, Tuman KJ, et al. Preoperative airway assessment: predictive value of a multivariate risk index. Anesth Analg. 1996;82:1197–204. doi: 10.1097/00000539-199606000-00017. [DOI] [PubMed] [Google Scholar]
- 28.Abe T, Kawakami Y, Sugita M, et al. Use of B-mode ultrasound for visceral fat mass evaluation: comparisons with magnetic resonance imaging. Appl Human Sci. 1995;14:133–39. doi: 10.2114/ahs.14.133. [DOI] [PubMed] [Google Scholar]