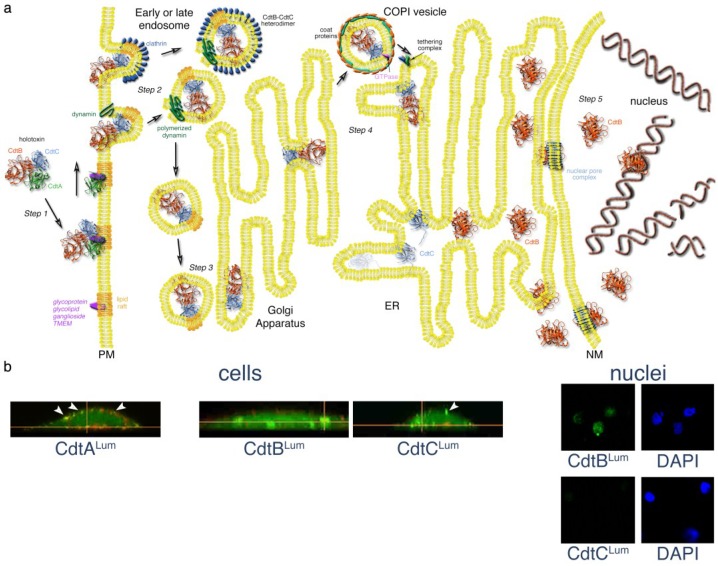
Figure 2.
Mechanistic model of Cdt trafficking in mammalian cells. (a) After binding to a putative receptor concentrated in membrane lipid rafts, the CdtA subunit remains on the surface. A CdtB-CdtC heterodimer enters the cell by either a clathrin-dependent or independent, receptor-independent endocytosis. Endosomal vesicles are formed by the polymerization of dynamin. Either early or late endosomes deliver the CdtB-CdtC complex to the Golgi. CdtB, the enzymatically-active subunit of the toxin, is moved from the Golgi to the ER by retrograde transport. COPI vesicles, originating from Golgi cisternae, encase the cargo by a process involving the binding of a small GTPase to the coat proteins. The COPI vesicles, directed to the ER by a tethering complex, fuse with the ER membrane. The CdtC protein is degraded in the ER. However, CdtB is translocated from the ER by either an endoplasmic reticulum-associated degradation (ERAD) or non-ERAD pathway. CdtB most likely enters the nucleus through a nuclear pore complex that recognizes a NLS sequence in the protein. However, it is possible that CdtB first enters the cytosol from the ER and then crosses the nuclear membrane through a nuclear pore complex. CdtB binds to DNA in the nucleus and initiates DNA damage by introducing single-and double-strand breaks. Details of the model are presented in the text. Elements of this model were modified from those presented in [48,49,50,51,52]; (b) Detection of the AaCdt subunits during intoxication using a fluorescein arsenical hairpin binding (FlAsH) dye technology and live-cell imaging. AaCdtA was detected only on the CHO cell surface, AaCdtC was present on both the cell surface (arrows) and inside the cells and AaCdtB was found inside the cells and in isolated nuclei. Cells were costained with Lumio™ Green and WGA-Alexa Fluor 555 (red). Nuclei were co-stained with Lumio and DAPI (4',6-diamidino-2-phenylindole). Details of the experiment are provided in the text and in [6].