Fig. 2.
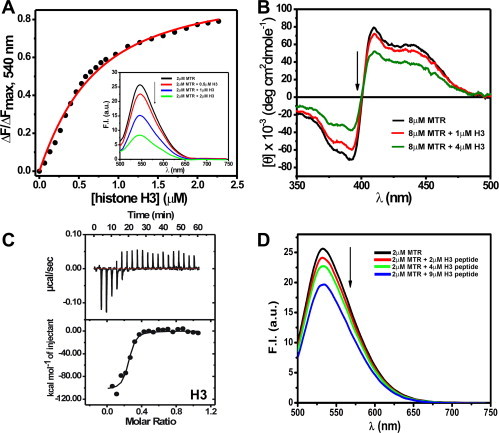
(A) Binding isotherm for the interaction of MTR with histone H3 in 10 mM Tris–HCl, pH 7.0 containing 150 mM NaCl at 25 °C obtained from steady state fluorescence spectroscopy. Inset shows the emission spectra of 2 μM MTR in absence (black) and presence of increasing concentrations (0.5 μM, red; 1 μM, blue; 2 μM, green) of human recombinant histone H3. λex = 470 nm. (B) Circular dichroism spectra of 8 μM MTR in 10 mM Tris–HCl, pH 7.0 containing 150 mM NaCl at 25 °C in absence (black) and presence of 1 μM (red) and 4 μM (green) histone H3 in the visible range. (C) ITC profile for the association of MTR with histone H3 at 25 °C in 10 mM Tris–HCl, pH 7.0 containing 150 mM NaCl. The lower panel contains the background heat subtracted fitted isotherm. Emission spectra of 2 μM MTR in 10 mM Tris–HCl, pH 7.0 containing 150 mM NaCl at 25 °C in absence (black) and in presence of (2 μM, red; 4 μM, green; 9 μM, blue) N-terminal tail peptide H3 (residues 1–21). λex = 470 nm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
