Fig. 3. Identification of candidate biomarkers of pediatric OSA.
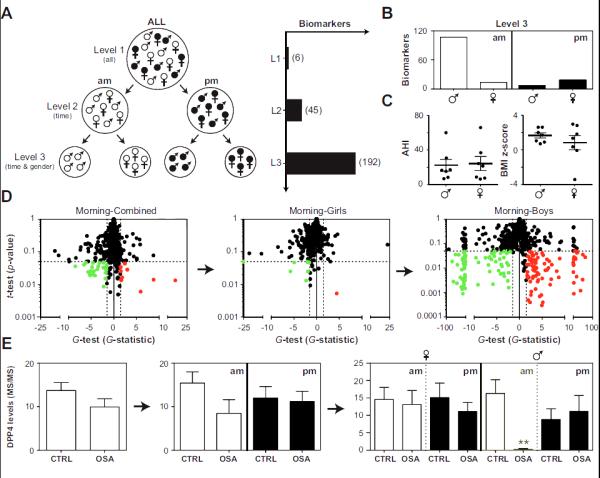
Morning (am) and bedtime (pm) samples were collected from children with and without OSA and subjected to LC-MS/MS. Panel A: Analysis of proteomic data was performed as follows: Level 1 (L1), morning and bedtime measurements were averaged and boys and girls were pooled; Level 2 (L2), analyses for morning and bedtime samples were conducted independently; Level 3 (L3) analyses for morning and bedtime samples were conducted independently in both boys and girls. The number of candidate biomarkers identified at each level is shown in parentheses. Panel B: Biomarkers detected in level 3 were split according to collection time and gender. Panel C: AHI and BMI z-scores in boys and girls with OSA. Panel D: A demonstration of the “gender effect” on global proteomic analysis (based on the t-test and G-test) of morning urine samples. Red, up-regulated in OSA; green, down-regulated in OSA; dashed lines confidence intervals (FDR < 5%). Panel E: Dipeptidyl peptidase 4 (DPP4) as an example of a specific biomarker for OSA in the morning samples of boys. Protein levels (mean ±SEMs) were determined by spectral counting. **, statistically significant based on the t-test and G-test.
