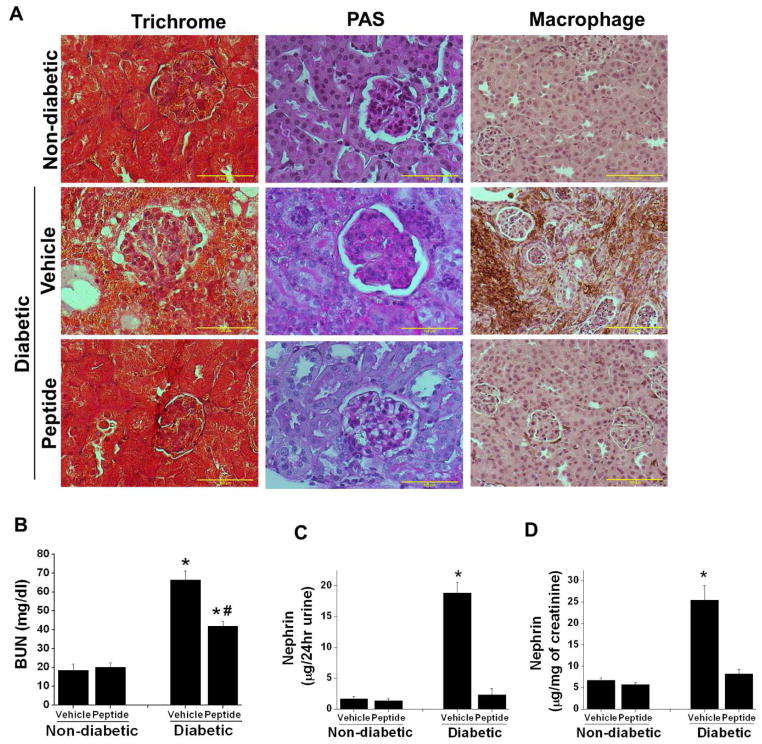
Figure 6.
Sema3A inhibitory peptide administration suppressed diabetes induced gloermulosclerosis, interstitial fibrosis, macrophage infiltration, kidney dysfunction. and nephrin-1 excretion in urine. DBA/2J mice made diabetic by administering low dose of STZ and non-diabetic animals received citrate buffer as described in Methods. 4 weeks after confirmation of diabetes animals received either 10μg/animal/day of scrambled peptide (vehicle) or sema3A complimentary inhibitory peptide (peptide) for another 6 weeks. Kidney tissue was processed for histopathology. A. Fibrosis and glomerular sclerosis was assessed by Masson’s trichrome staining and PAS staining. Diabetes induced extensive damage in parenchyma and glomerulosclerosis in vehicle treated mice which was suppressed in sema3A peptide treated diabetic animal kidney. In addition, diabetes induced significant increase in macrophage infiltration in vehicle treated mice kidney which was reduced in sema3A antagonist peptide treated diabetic mice kidney. Scale bar: 100 μM. B. Kidney function was measured by quantifying blood urea nitrogen (BUN). Administration of sema3A inhibitory peptide significantly reduced diabetes induced kidney dysfunction. *, p<0.001 vs. non-diabetic animals. #, p<0.05 vs. Vehicle treated diabetic animals. n=6–8. C & D. Podocyte injury marker nephrin-1 was quantified as described in Methods. Diabetes induced a large increase in nephrin-1 excretion in vehicle treated diabetic mice as compared to controls which was largely suppressed in peptide treated diabetic mice. *, p<0.001 vs. all other groups. n=6.