Abstract
Traditional adipose tissue transplantation has unpredictable viability and poor absorption rates. Recent studies have reported that treatment with platelet-rich plasma (PRP), adipose-derived stem cells (ASCs), and stromal vascular fraction (SVF) are related to increased survival of grafted adipose tissue. This study was the first simultaneous comparison of graft survival in combination with PRP, ASCs, and SVF. Adipose tissues were mixed with each other, injected subcutaneously into the back of nude mice, and evaluated at 4, 8, and 12 weeks. Human adipocytes were grossly maintained in the ASCs and SVF mixtures. Survival of the adipose tissues with PRP was observed at 4 weeks and with SVF at 8 and 12 weeks. At 12 weeks, volume reduction in the ASCs and SVF mixtures were 36.9% and 32.1%, respectively, which were significantly different from that of the control group without adjuvant treatment, 51.0%. Neovascular structures were rarely observed in any of the groups. Our results suggest that the technique of adding ASCs or SVF to transplanted adipose tissue might be more effective than the conventional grafting method. An autologous adipose tissue graft in combination with ASCs or SVF may potentially contribute to stabilization of engraftment.
Graphical Abstract
Keywords: Adipose Tissue, Tissue Graft, Platelet-Rich Plasma, Stem Cell
INTRODUCTION
A number of methods have been introduced to correct soft tissue defects or asymmetric contours in reconstructive and regenerative surgery. Autologous fat graft is one of the best-documented and has become increasingly popular (1, 2, 3, 4).
Although a fat graft is a simple procedure without a foreign body response at the recipient site and results in less morbidity at the donor site, the unpredictable survival of grafted fat is a major drawback, with survival rates varying from 30% to 80%. Thus, there is great value in increasing the survival rates of fat graft techniques (5, 6, 7). Considerable efforts have been made to improve the survival rate at each step of the procedure, from lipoaspiration to transplantation. Although these enhanced harvesting techniques have significantly reduced the resorption rate, rates remain unsatisfactory.
Therefore, several trials have been initiated targeting the transplantation step in order to improve the survival of grafted fat. Platelet-rich plasma (PRP) from peripheral blood, which was introduced by Whitman et al. (8), has a higher concentration of platelets than normal plasma (approximately 150-400×103 cell/µL). Choi et al. (9) suggested that PRP could improve adipocyte survival when concurrently injected because it contains numerous growth factors. In addition, due to the development of tissue engineering, isolated and cultured mesenchymal stem cells could be mixed into the fat graft in order to promote survival (10). Adipose-derived stem cells (ASCs), which are mesenchymal stem cells similar to those found in the bone marrow, differentiate into a wide range of cells (11) and can be extracted and cultured from a lipoaspirate. These stem cells are the progenitors of adipocytes, osteocytes, and chondrocytes (12, 13, 14) and also secrete angiogenic growth factors, which play a key role in stabilizing grafted tissues. It is relatively simple to obtain large quantities of lipoaspirate using liposuction, and the defect at the donor site is acceptable. The survival rate of grafted fat is enhanced when ASCs are mixed with adipose cells (15, 16).
However, the clinical application of stem cells is quite difficult. Many countries mandate operating systems in an approved culture facility according to patient safety protocols, and in some countries, government approval is mandatory (17). Moreover, as the processes underlying ASCs culture take a considerable amount of time, they cannot immediately be applied clinically. Unlike cultured and relatively homogeneous ASCs, an uncultured heterogeneous stromal vascular fraction (SVF) is easily prepared for clinical use without time-consuming culture procedures. Both can be extracted from lipoaspirates, but SVF contains a stromal component, including granulocyte, monocyte, lymphocyte, endothelial cells, pericytes and fibroblast-like interstitial cells (18). Additionally, it has stem and progenitor cells, termed ASCs. The ASCs are derived from this SVF sub-population. The SVF is the centrifuged, non-expanded, and suspended cell pellet. Supplementation of fat grafts with SVF is an emerging technique in the field of regenerative medicine that is used to improve graft reliability.
Although several studies of the effects of PRP, ASCs, and SVF on fat graft survival have been conducted independently, their impact is still not fully understood. Therefore, the aim of this study was to evaluate the effects of adjuvant PRP, ASCs, and SVF on the survival of fat grafts.
MATERIALS AND METHODS
Extraction of adipose tissue
Human adipose tissues were obtained from three patients who underwent cosmetic liposuction procedures. All patients were females, with ages ranging from 27 to 34 yr. They were healthy and were not taking any medications. Their body mass indexes ranged from 24 to 29 kg/m2. Informed consent was obtained from all patients regarding liposuction and blood sampling before fat harvest under an experimental protocol approved by the Institutional Animal Care and Use Committee of Korea University Ansan Hospital (AS-11003-002).
The donor sites were chosen from the lower abdomen, hip, and thigh. Anesthetics were introduced through intravenous injection of ketamine, 1.0-2.0 mg/kg (Huons Co., Seoul, Korea); midazolam, 0.1-0.2 mg/kg (Bukwang Pharm. Co., Seoul, Korea); and propofol, 1.5-2.5 mg/kg (Dongkook Co., Seoul, Korea). Heart rate and oxygen saturation were monitored. A tumescent solution consisting of a mixture of 1,000 mL of Hartmann's solution (JW Pharm. Co., Seoul, Korea), 20 mL of 2% lidocaine (Huons Co.), and 1 mL of epinephrine (Daihan Pharm. Co., Seoul, Korea) resulting in a 1:1,000,000 concentration of epinephrine was prepared and was evenly injected into the patient's donor sites to induce anesthesia using a 10 mL syringe.
After waiting 10 min until the tumescent solution penetrated into the tissue, a blunt-tip 3-mm Mercedes catheter (Byron Medical Inc, Tucson, AZ, USA) was connected to a 10-mL Luer-lock (Becton, Dickinson and Co., Franklin Lakes, NJ, USA) syringe. Using this device, a negative pressure 1-2 mL vacuum was applied to aspirate the adipose tissue. In order to minimize destruction of the adipose tissue, the vacuum space in the 10 mL syringe was limited to less than 2 mL. The syringe loaded with the aspirated fat tissue was capped so that the syringe was airtight and kept upright. The adipose tissue was centrifuged at 800×g for three minutes to separate supernatant fluid from the blood cells, tumescent solution, and water in the lower layers, which were subsequently removed. The fluid in the middle layer containing the adipose cells was collected.
Preparation of SVF
The extracted adipose tissue was rinsed with phosphate-buffered saline (PBS, Gibco, Carlsbad, CA, USA) to remove blood cells, tumescent solution, and water before undergoing treatment with 0.05% collagenase type IA (Sigma-Aldrich, St Louis, MO, USA) at 37℃ for 60 min. After degradation, the activity of the collagenase was neutralized with 10% fetal bovine serum (FBS, Gibco), which was added in the same amount as the collagenase. The treated cells were centrifuged at 400× g for 10 min. Supernatant fluid was removed after centrifugation. The pellets were then resuspended in Dulbecco's modified Eagle's medium (DMEM, Gibco) with 1% antibiotics-antimycotics. The cell suspension was filtered through 70 µm nylon filters, and the sediment was the uncultured SVF containing ASCs. After the mixture was dyed with trypan blue, evenly distributed SVF with 3×105 cells/20 µL were prepared and measured using a hemocytometer.
Preparation of ASCs
To separate ASCs from the SVF, the prepared SVF was placed in a 75-cm2 culture flask and incubated at 37℃ for 24 hr in an atmosphere of 5% carbon dioxide humidified air. DMEM with 10% FBS, epidermal growth factor, and beta-fibroblast growth factor was used as the culture medium. After 24 hr, the medium was discarded and the SVFs were washed again with PBS to remove dead cells. Sufficient amounts of ASCs (80% of the flask area) were obtained as subcultures three times by replacing the DMEM medium every three days. The ASCs were prepared using trypan blue to reach a cell density of 3×105 cells/20 µL, which is similar to that of the SVF.
Preparation of PRP
PRP was prepared by collecting 12 mL of blood from the same patients who participated in the lipoaspiration. Four milliliters of blood was dispensed into each blood collection tube containing 1.2 mL of the anticoagulant 10% sodium citrate. The blood samples were centrifuged at 1,700 rpm for 10 min resulting in three layers. The top layer consisting of plasma and the intermediate layer composed of a buffy coat were separated from the red blood cell fraction in the lower layer and were collected into test tubes. Then, the tubes were centrifuged at 800×g for another 10 min to separate the PRP precipitate from the platelet-poor plasma in the floating layer. PRP with a platelet density of 1.2×108 cells/20 µL was used in this study.
Experimental design of the fat graft
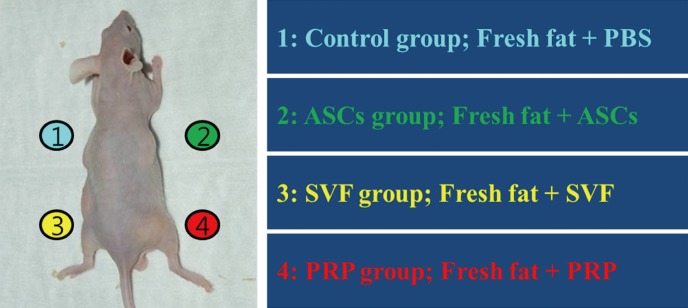
Immunosuppressed nude mice were used in the experiment to prevent graft rejection of human fat tissue. The mice were six-week-old BALB/c (n=18) and were all females weighing approximately 20 g. Both control and experimental grafts were simultaneously placed on the backs of the same mouse to minimize inter-individual variation. Freshly isolated SVF, cultured ASCs, and purified PRP were added to the adipose tissue. Initially, 20 µL of SVF, ASCS, and PRP were added to 80 µL of PBS. Then, 100 µL of the mixture was mixed with 300 µL of human fat tissue. The control group was prepared with 100 µL of PBS and 300 µL of human fat tissue in a similar manner. After gentle mixing and waiting for 10 min, the cell-supplemented fat was then transferred into 10-mL Luer-Lock syringes for placement of the graft. The mice were anesthetized using an intraperitoneal injection of 0.2 mL of ketamine. The mixtures of fat and adjuvants were subcutaneously transplanted into the backs of the mice in the panniculus carnosus layer, where there is no fatty layer, and just deep to the panniculus adiposus layer in order to avoid disturbing murine fat tissue. The back of the mice were divided into quadrants, and each graft mixture was injected using an 18-gauge needle into a separate quadrant, with care being taken not to intrude into the other quadrants (Fig. 1). The nude mice were then fed clean forage and water under aseptic conditions. Six mice were sacrificed at 4, 8, and 12 weeks, respectively, after transplantation, and the grafted tissues were harvested.
Fig. 1.
Schematic of the study procedures. The mouse's back was divided into four quadrants. Fresh human adipose tissues with ASCs, SVF, and PRP were injected subcutaneously. PBS, phosphate-buffered saline; ASCs, adipose-derived stem cells; SVF, stromal vascular fraction; PRP, platelet-rich plasma.
Macroscopic analysis of grafted fat tissue
To analyze the results of the fat graft, we chose to evaluate volume rather than weight. As there was no method to directly measure the volume of the grafted fat tissue, it was indirectly calculated using Archimedes' principle. After washing the grafted tissues with saline, they were placed in a 2-mL syringe containing 1 mL of saline. The volume of grafted tissue was determined by measuring the increase in saline volume. The specimens were divided in half and each half underwent histologic and immunohistochemical staining.
Histological and immunohistochemical analysis
At each period, the isolated fat tissue was fixed by soaking in a 10% buffered formalin solution and embedded in paraffin wax for histological examination. The mid-portion of the paraffin blocks containing the cell clusters were sectioned at a thickness of 5 µm on a microtome. The sectioned slices were stained with hematoxylin and eosin, mounted on a glass slide, and examined using light microscopy (Olympus BX51, Olympus Optical Co., Tokyo, Japan) at 100× magnification.
The degree of neo-angiogenesis in the tissue was examined by immunohistochemical staining using CD31, a vascular endothelial cell-specific marker. The samples were embedded into glass slides, as described above. The glass slides were placed at 60℃ for 1 hr and deparaffinized with xylene. After immersion in 0.01 M citrate solution, the slides were heated in the microwave for 10 min and washed with saline. They were then fixed with 3% H2O2 and treated with CD31 primary mouse antibodies with human reactivity (Gibco) at 4℃ for 24 hr. Next, the slides were treated with CD31 secondary anti-mouse antibodies (Gibco), streptavidin, and diaminobenzene using a Cap-Plus™ Detection Kit (Gibco). The nuclei were stained with hematoxylin, and the slides were observed microscopically at 200× magnification.
Statistical analysis
The mean and 95% confidence interval (CI) of the surviving fat volume were calculated. Comparisons of the survival rate among each experimental group and time interval were performed. Data were analyzed using statistical analysis software (SPSS, SPSS Inc., Chicago, IL, USA). A Kruskal-Wallis test and Mann-Whitney u test were used. The level of statistical significance was determined as P<0.05, and Tukey contrasts were used for multiple comparisons of means.
RESULTS
Macroscopic findings
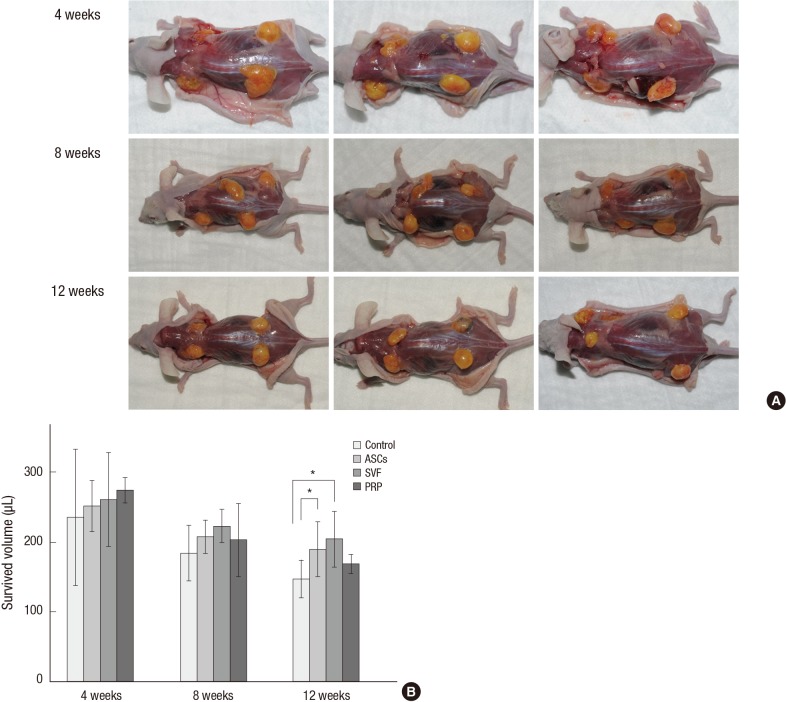
All grafted human fat tissues were soft, non-migratory, and yellow healthy adipose tissue. Neovascular structures were found after eight weeks post-transplantation (Fig. 2A). The grafts were placed in the panniculus carnosus layer and encapsulated within thin connective tissue; therefore, it was easy to separate them from the adjacent murine tissues. There were no signs of hematoma, inflammatory tissue contractures, or tissue breakdown in the recipient sites.
Fig. 2.
Surviving volume of grafted human fat tissue. (A) The macroscopic appearance showed that neovasculature structures were observed in the adjuvant groups after 8 weeks. (B) Volumes of the surviving adipose tissues. For the ASCs and SVF groups, the mean volumes were significantly greater than that of the control at 12 weeks. Mean and 95% CI are shown (*P < 0.05). ASCs, adipose-derived stem cells; SVF, stromal vascular fraction; PRP, platelet-rich plasma.
Volume analysis of the grafted fat tissue
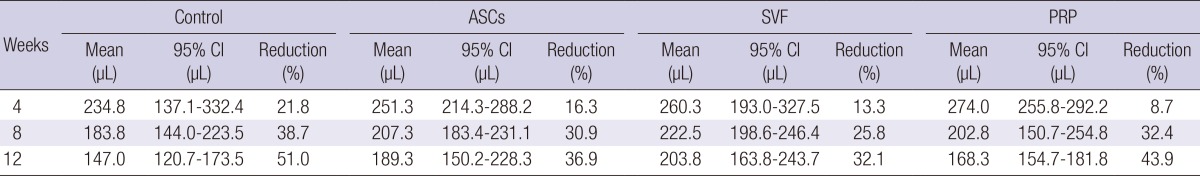
At four weeks after the fat graft, the mean of the total volumes of surviving fat tissue were 234.8 µL in the control group, 251.3 µL in the ASCs group, 260.3 µL in the SVF group, and 274.0 µL in the PRP group. Shortly after the transplant, the PRP group had the largest fat volume. The surviving fat volume was largest in the SVF group and was 222.5 µL at eight weeks. The means of the final volumes in each group at 12 weeks were: 147.0 (95% CI, 120.7-173.3) µL in the control group, a 51.0% reduction; 189.3 (95% CI, 150.2-228.3) µL in the ASCs group, a 36.9% reduction; 203.8 (95% CI, 163.8-243.7) µL in the SVF group, a 32.1% reduction; and 168.3 (95% CI, 154.7-181.8) µL in the PRP group, a 43.9% reduction (Table 1).
Table 1.
Comparison of the mean volumes of surviving adipose tissue in the control, ASCs, SVF, and PRP groups. ASCs, adipose derived stem cells; SVF, stromal vascular fraction; PRP, platelet-rich plasma
ASCs, adipose-derived stem cells; SVF, stromal vascular fraction; PRP, platelet-rich plasma.
All experimental groups that received adjuvants showed greater retention of grafted fat than the control group. At 12 weeks after the fat graft, the volume reductions in the SVF and ASCs groups were significantly different from that of the control group, P=0.008 and P=0.047, respectively, with post-hoc Tukey corrections. The volume reductions in the SVF and ASCs groups did not significantly differ from each other (P=0.734). The fat volume in the PRP group was a little larger than that of the control group, although this difference was not significant (P=0.46). In addition, the fat volume in the PRP group was not significantly different from that of the SVF group (P=0.105) at 12 weeks. There were no significant differences in survival rates among the groups at four and eight weeks after the fat grafts (Fig. 2B).
Microscopic examination of the grafted fat tissue
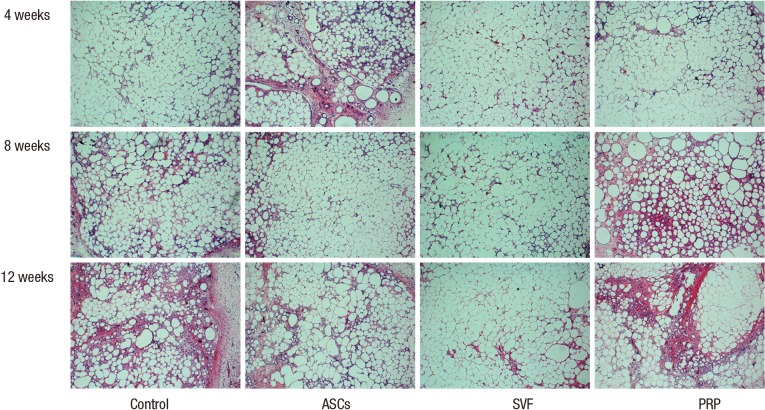
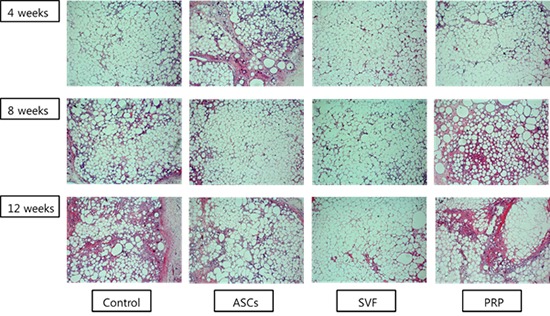
At four weeks follow-up, the fat cells in all groups generally had maintained their shape. Some lipid vacuoles were found due to atrophy of fat cells in the PRP and control groups at eight weeks. On the other hand, most fat cells maintained their dense forms in the ASCs and SVF groups. In particular, the SVF group showed greater compaction of its adipose cells at 12 weeks than the ASCs group (Fig. 3).
Fig. 3.
Representative images from hematoxylin and eosin staining. In the ASCs and SVF groups, the structure of the fat cells was generally maintained at 8 weeks after transplantation. At 12 weeks, the cells in the SVF group showed a more stable form than in any of the other groups (100×). ASCs, adipose-derived stem cells; SVF, stromal vascular fraction; PRP, platelet-rich plasma.
Immunohistochemical analysis
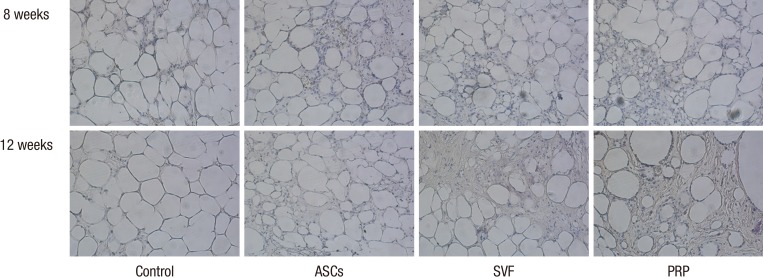
Immunohistochemical analysis using CD31 was performed at 8 and 12 weeks after adipose tissue transplantation. Fat cells were found to survive in greater densities in the ASCs and SVF groups than in the PRP and control groups, as demonstrated with hematoxylin and eosin staining. However, CD31 antibodies with human reactivity did not readily stain vascular endothelial cells. No vasculitis or inflammatory cells were found near the newly-formed vascular structures in the fat graft specimens (Fig. 4).
Fig. 4.
Representative images of CD31 antibody staining. Neovasculature structures were observed on the side with adjuvants at all time points. However, vascular endothelial cells were scarcely stained by CD31 antibodies with human reactivity (200×). ASCs, adipose-derived stem cells; SVF, stromal vascular fraction; PRP, platelet-rich plasma.
DISCUSSION
We performed the present study to evaluate the individual contribution of ASCs, SVF, and PRP to the increased survival of transplanted adipose tissue in an immunocompromised mouse model. This study addresses the effects of ASCs and SVF when they are individually used as adjuvants.
In this experimental model, we controlled the conditions of the fat graft. We used human ASCs and adipose tissue instead of those derived from mice. For transplantation, autologous cells are generally preferred to allogenic because of the potential risk of a host immune response (19). In this study, we selected immunocompromised mice to prevent the graft rejection that is associated with xenografts. Aspirated adipose tissues were minimally processed during preparation and the mixtures were immediately injected into the backs of the mice to minimize potential damage to the grafted tissue (20). The mixtures were transplanted into an area where mouse adipose tissue and muscles are scarce. The control and experimental groups were designed to be on the same nude mouse to decrease inter-individual variation bias and thus support our study's goal to focus on the effects of adjuvant treatment and its interactions with the grafted tissue.
We observed an 8.73% volume reduction four weeks after the fat graft in the PRP group. The ASCs group and the SVF group showed volume reductions of 16.3% and 13.3%, respectively. However, 8 weeks and 12 weeks after graft placement, the SVF group outcome was superior to the other groups. The ASCs group showed a significant difference from the control group at 12 weeks. This indicated that PRP contributes to the acceleration of early graft stabilization, while ASCs and SVF contribute to late maturation. PRP is considered a rich source of growth factors and has been reported to improve the fat survival rate by accelerating neo-angiogenesis through release of platelet-derived growth factor, vascular endothelial cell growth factor, transforming growth factor, epithelial growth factor, and hepatocyte growth factor (9). However, in the case of PRP, it is thought that the lifespan of platelets in the wound and the period during which growth factors have a direct effect are shorter than seen with other adjuvants (21). In addition, PRP has limited potential for cell differentiation because it is mainly composed of platelets.
Successful tissue transplantation depends on providing an adequate environment to maintain the tissue's biologic requirements. Both ASCs and SVF include mesenchymal stem cells that have the ability to differentiate into multiple lineages of mature cells. ASCs can differentiate into adipogenic, osteogenic, cartilaginous, and neurogenic cells by lineage-specific paracrine growth factors (9). These cells secrete multiple angiogenic and adipogenic growth factors, such as vascular endothelial growth factor and hepatocyte growth factor, to help grafts incorporate into the recipient tissue (16, 22). Furthermore, SVF contains an abundant stromal component, which is a major difference from ASCs. In the present study, although there was no significant difference in the amount of surviving adipose tissue, a greater amount was observed in the SVF group than in the ASCs group. It is possible that the stromal component contributes to enhancement of the paracrine effect of ASCs on the grafted material for the establishment of neovascular connections. Matsuda et al. (23) reported that ASCs survived and expressed growth factors to promote tissue growth in an appropriate extracellular environment. Another hypothesis is that the stromal component is related to the migration of ASCs to an oxygenated region, which was reported by Dong et al. (24). Therefore, the present findings suggest that SVF and ASCs are more likely to enhance the survival of transplanted adipose tissue by stabilizing engraftment.
Unlike the ASCs, the preparation of SVF does not require a culturing step and SVF can be easily and efficiently obtained in large quantities (e.g. 1L). On the other hand, the clinical use of ASCs is limited for the following reasons: the requirement for government approval before use of tissue-engineered stem cells, the extensive periods of time needed to culture cells, the uncertainty of the carcinogenic effect, and the potential for contamination.
Regarding the simultaneous transplantation of adipose tissue and adjuvants in the present study, we expected that once the growth factors of the adjuvants acted on the adipose tissue, neo-angiogenesis would occur. At 8 and 12 weeks after transplantation, we observed a number of neovascular structures in the SVF, ASCs, PRP, and control groups, in order of decreasing frequency. We hypothesized that neo-angiogenesis would be more pronounced in the adjuvant groups than in the control groups, and the neovascular structures could be differentiated from human ASCs. However, our study demonstrated that the newly formed vessels were not from the human tissue, but were from the recipient's tissue. We found no remarkable signals in the CD31 immunohistochemical study, which was targeted to vascular endothelial cells with human reactivity. This result revealed that the grafted stem cells did not differentiate into vascular endothelial cells, although they may have enhanced neo-angiogenesis leading to development of vessels near the graft site.
In conclusion, we observed that ASCs and SVF had a positive effect on the survival of transplanted adipose tissue when mixed as adjuvants. Further studies are needed to elucidate the mechanisms by which ASCs and SVF contribute to increased graft tissue survival, and to investigate the role of the stromal component of SVF.
Footnotes
This study was supported by a grant from Korea University, Seoul, Korea.
The authors have no potential conflicts of interest to disclose.
References
- 1.Sommer B, Sattler G. Current concepts of fat graft survival: histology of aspirated adipose tissue and review of the literature. Dermatol Surg. 2000;26:1159–1166. [PubMed] [Google Scholar]
- 2.Illouz YG. Present results of fat injection. Aesthetic Plast Surg. 1988;12:175–181. doi: 10.1007/BF01570929. [DOI] [PubMed] [Google Scholar]
- 3.Moseley TA, Zhu M, Hedrick MH. Adipose-derived stem and progenitor cells as fillers in plastic and reconstructive surgery. Plast Reconstr Surg. 2006;118:121s–128s. doi: 10.1097/01.prs.0000234609.74811.2e. [DOI] [PubMed] [Google Scholar]
- 4.Ramon Y, Shoshani O, Peled IJ, Gilhar A, Carmi N, Fodor L, Risin Y, Ullmann Y. Enhancing the take of injected adipose tissue by a simple method for concentrating fat cells. Plast Reconstr Surg. 2005;115:197–201. discsussion 2-3. [PubMed] [Google Scholar]
- 5.Moore JH, Jr, Kolaczynski JW, Morales LM, Considine RV, Pietrzkowski Z, Noto PF, Caro JF. Viability of fat obtained by syringe suction lipectomy: effects of local anesthesia with lidocaine. Aesthetic Plast Surg. 1995;19:335–339. doi: 10.1007/BF00451659. [DOI] [PubMed] [Google Scholar]
- 6.Shiffman MA, Mirrafati S. Fat transfer techniques: the effect of harvest and transfer methods on adipocyte viability and review of the literature. Dermatol Surg. 2001;27:819–826. doi: 10.1046/j.1524-4725.2001.01062.x. [DOI] [PubMed] [Google Scholar]
- 7.Pu LL, Cui X, Fink BF, Cibull ML, Gao D. The viability of fatty tissues within adipose aspirates after conventional liposuction: a comprehensive study. Ann Plast Surg. 2005;54:288–292. [PubMed] [Google Scholar]
- 8.Whitman DH, Berry RL, Green DM. Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg. 1997;55:1294–1299. doi: 10.1016/s0278-2391(97)90187-7. [DOI] [PubMed] [Google Scholar]
- 9.Choi J, Minn KW, Chang H. The efficacy and safety of platelet-rich plasma and adipose-derived stem cells: an update. Arch Plast Surg. 2012;39:585–592. doi: 10.5999/aps.2012.39.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang JD, Choi DS, Cho YK, Kim TK, Lee JW, Choi KY, Chung HY, Cho BC, Byun JS. Effect of amniotic fluid stem cells and amniotic fluid cells on the wound healing process in a white rat model. Arch Plast Surg. 2013;40:496–504. doi: 10.5999/aps.2013.40.5.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim YJ, Jeong JH. Clinical application of adipose stem cells in plastic surgery. J Korean Med Sci. 2014;29:462–467. doi: 10.3346/jkms.2014.29.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 13.Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 14.Salibian AA, Widgerow AD, Abrouk M, Evans GR. Stem cells in plastic surgery: a review of current clinical and translational applications. Arch Plast Surg. 2013;40:666–675. doi: 10.5999/aps.2013.40.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 17.You HJ, Han SK. Cell therapy for wound healing. J Korean Med Sci. 2014;29:311–319. doi: 10.3346/jkms.2014.29.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eun SC. Composite tissue allotransplantation immunology. Arch Plast Surg. 2013;40:141–153. doi: 10.5999/aps.2013.40.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith P, Adams WP, Jr, Lipschitz AH, Chau B, Sorokin E, Rohrich RJ, Brown SA. Autologous human fat grafting: effect of harvesting and preparation techniques on adipocyte graft survival. Plast Reconstr Surg. 2006;117:1836–1844. doi: 10.1097/01.prs.0000218825.77014.78. [DOI] [PubMed] [Google Scholar]
- 21.Yun JH, Yoo JH, Choi SH, Lee MH, Lee SJ, Song SU, Oh NS. Synergistic effect of bone marrow-derived mesenchymal stem cells and platelet-rich plasma on bone regeneration of calvarial defects in rabbits. Tissue Eng Regen Med. 2012;9:17–23. [Google Scholar]
- 22.Liu M, Guo L, Liu Y, Pei Y, Li N, Jin M, Ma L, Li Z, Sun B, Li C. Adipose stromal-vascular fraction-derived paracrine factors regulate adipogenesis. Mol Cell Biochem. 2014;385:115–123. doi: 10.1007/s11010-013-1820-6. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda K, Falkenberg KJ, Woods AA, Choi YS, Morrison WA, Dilley RJ. Adipose-derived stem cells promote angiogenesis and tissue formation for in vivo tissue engineering. Tissue Eng Part A. 2013;19:1327–1335. doi: 10.1089/ten.tea.2012.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong Z, Peng Z, Chang Q, Lu F. The survival condition and immunoregulatory function of adipose stromal vascular fraction (SVF) in the early stage of nonvascularized adipose transplantation. PLoS One. 2013;8:e80364. doi: 10.1371/journal.pone.0080364. [DOI] [PMC free article] [PubMed] [Google Scholar]