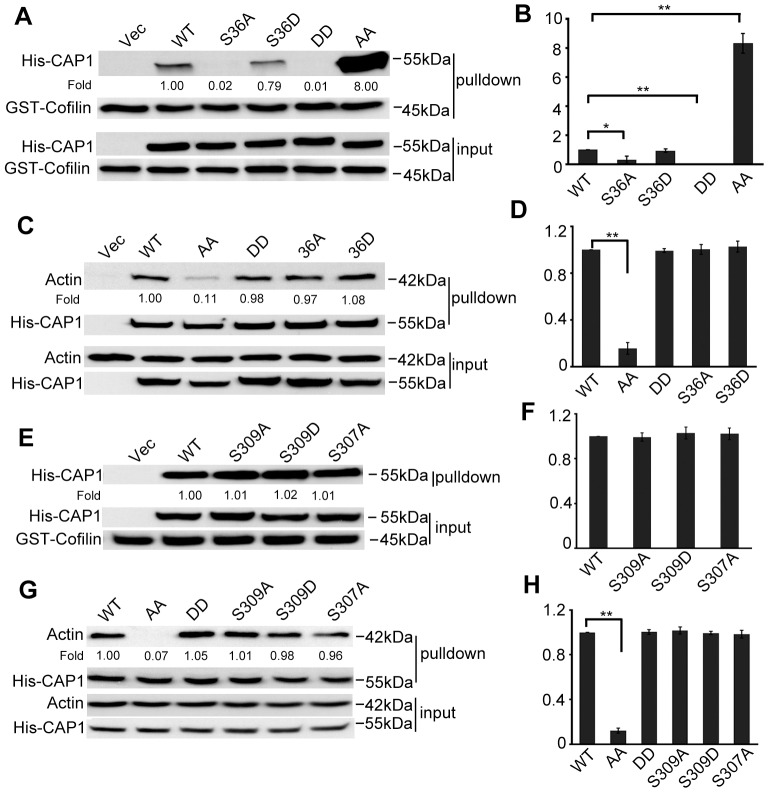
Fig. 3.
Altered binding of cofilin and actin for the S307/S309 mutants, and evidence that S307 and S309 function in tandem. (A) GST-cofilin pull-down revealed loss of cofilin binding of the DD mutant, whereas the AA mutant showed drastically increased cofilin binding. Equal amounts of GST-cofilin bound to glutathione beads (∼10–20 µg) were added to lysates containing ∼400 µg total protein harvested from CAP1 knockdown HeLa cells re-expressing WTCAP1 or the mutants. Tagged CAP1 co-precipitated with cofilin was detected with a 6×His antibody and GST-cofilin was detected with a cofilin antibody. (B) Quantification of results shown in A. Three independent experiments of GST–cofilin pull-down were measured by densitometry, and the results were analyzed and shown in the graph; error bars represent ±s.e.m.; *P<0.05, **P<0.01. (C) Actin co-precipitation assays reveal reduced actin binding for the AA mutant. Cell lysates prepared from CAP1-knockdown HeLa cells re-expressing WTCAP1 or the mutants were incubated with Ni-NTA beads, and the beads were precipitated and washed with lysis buffer containing 20 mM imidazole. Samples were resolved on SDS-PAGE, and detected with antibodies against actin and 6×His in western blotting. (D) Quantification of results shown in C. Three independent experiments were measured by densitometry, results were analyzed and are shown in the graph; error bars represent ±s.e.m.; **P<0.01. (E,F) A single S307A or S309 mutation did not mimic the drastic increase in cofilin binding, the experiments and data analysis were done in a similar manner as described for A and B. (G,H) A single S307A or S309A mutation did not mimic reduced actin binding. Experiments and data analysis were done as described for C and D; **P<0.01. In panels B, D, F and H, the y-axis represents the capability for cofilin and/or actin binding; the value for WT CAP1 was set at 1, and values for the mutants are relative to those of WT CAP1.