Abstract
Left ventricular free wall rupture (LVFWR) is a grave complication of acute myocardial infarction. Acutely, it has an extremely high rate of mortality, especially if undetected. Chronically, there is paucity of data on how to manage the pathology, especially if detected as an incidental finding. We present a unique case of initially undetected LVFWR in a patient who presented with repeated chest pain. Cardiac MRI enabled the diagnosis and localisation of the original site of LVFWR and provided highly relevant volume calculations. Measurement of both absolute and virtual volumes indicated that aneurysm resection, including the original site of rupture, was surgically feasible without reducing postoperative stroke volume.
Background
Left ventricular free wall rupture (LVFWR) is a catastrophic complication of acute myocardial infarction (AMI). Early intervention can be life-saving; therefore, an awareness of its clinical features is essential to delivering prompt and decisive management. We present a unique case of LVFWR with marked pseudoaneurysm formation that went initially undetected. Cardiac MR (CMR) subsequently identified the true extent of myocardial injury and a sealed LVFWR. Furthermore, CMR directed treatment towards surgery by demonstrating that surgical removal of the large aneurysm was feasible without compromising stroke volume (SV). This case illustrates the unique role of CMR in the patient care pathway and reinforces the importance of a high index of suspicion for this serious complication of AMI.
Case presentation
A 71-year-old woman presented to a regional cardiac centre for primary coronary intervention (PCI). Her medical history included hypertension, osteoarthritis, a cholecystectomy and a mastectomy for breast cancer 20 years prior.
She was transferred from home via ambulance with a short history of severe chest pain radiating to the right shoulder. This was described as an ache that made her feel short of breath and clammy. In the ambulance and on arrival to the catheter laboratory her ECG demonstrated marked ST elevation in the inferior leads (figure 1). The patient subsequently underwent emergency coronary angioplasty for a ST elevation myocardial infarction (MI) and two overlapping drug-eluting stents were placed in the right coronary artery.
Figure 1.
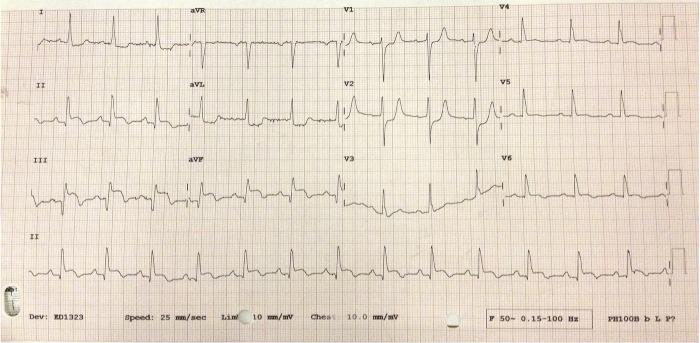
ECG on presentation 2 weeks post-primary coronary intervention. ST elevation in leads II, III and aVF, reciprocal ST depression in I, aVL, V1–3, T wave inversion in V5 and V6.
Two weeks after discharge, the patient was again admitted to hospital with chest pain. The nature of the pain and the associated symptoms were identical as before, they again came on at rest and were not relieved by the patient's newly prescribed GTN spray. The ECG revealed resolving but persisting ST elevation and the serum troponin I was 305 ng/L, significantly lower than the troponin post-PCI of 9262 ng/L 2 weeks earlier. This was therefore not consistent with an acute coronary event. A repeat echocardiogram revealed a minimal pericardial effusion; the pain settled with simple analgesia and the patient was discharged home with a provisional diagnosis of pericarditis. A follow-up outpatient echocardiogram was arranged to monitor the small effusion.
Investigations
The follow-up echocardiogram 3 months later demonstrated a marked change in anatomy. The left ventricle was dilated with a new 5.2×4.9 cm aneurysmal chamber communicating with the inferolateral wall of the left ventricle (figure 2). Blood flow was identified within this chamber demonstrated by colour-flow Doppler in which echogenic material, consistent with thrombus, was also present. The patient was therefore admitted and a cardiac MRI was arranged.
Figure 2.
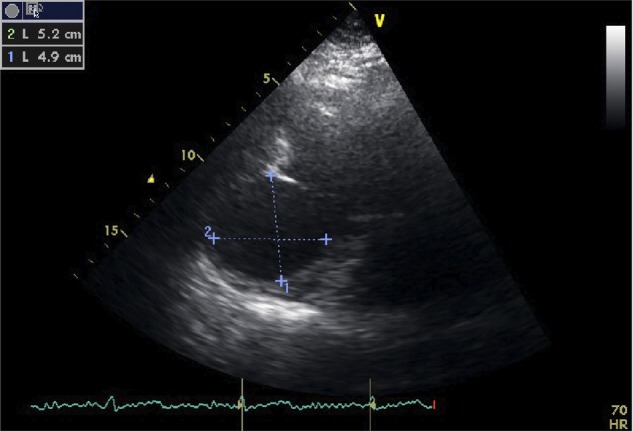
Echocardiographic images, three-chamber view. Aneurysmal sac protruding from the posteroinferior wall of the left ventricle measuring 5.2×4.9 cm.
The CMR revealed a more complex pathology. The CMR was performed on a 1.5 T Siemens Avanto scanner equipped with a dedicated 32-element cardiac coil system. It revealed a severely depressed ejection fraction (EF) of 24%, largely secondary to SV off-loading into a large pseudoaneurysm (videos 1 and 2) with layered thrombus formation over the inferolateral wall segments. Critically the ventricular thrombus extended outside of the aneurysmal wall into the pericardial space indicating a sealed LVFWR (figure 3).
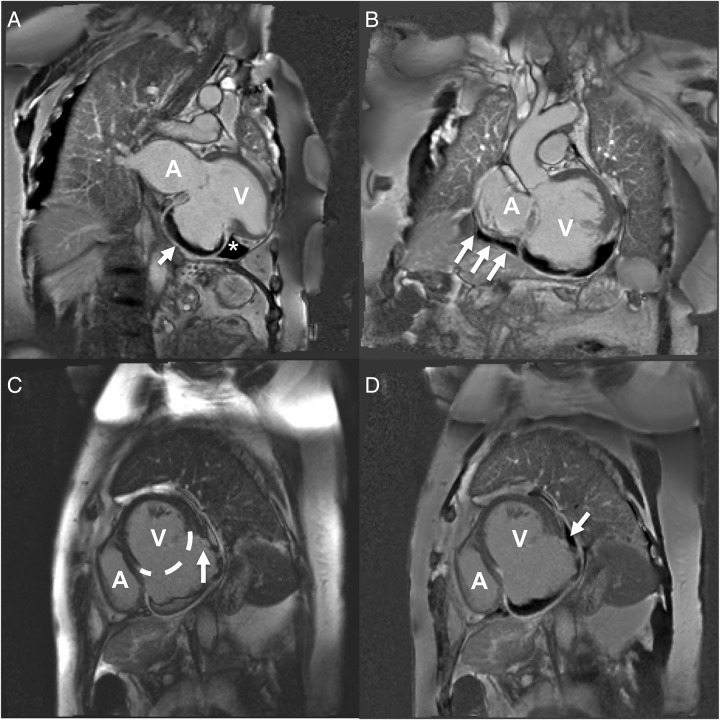
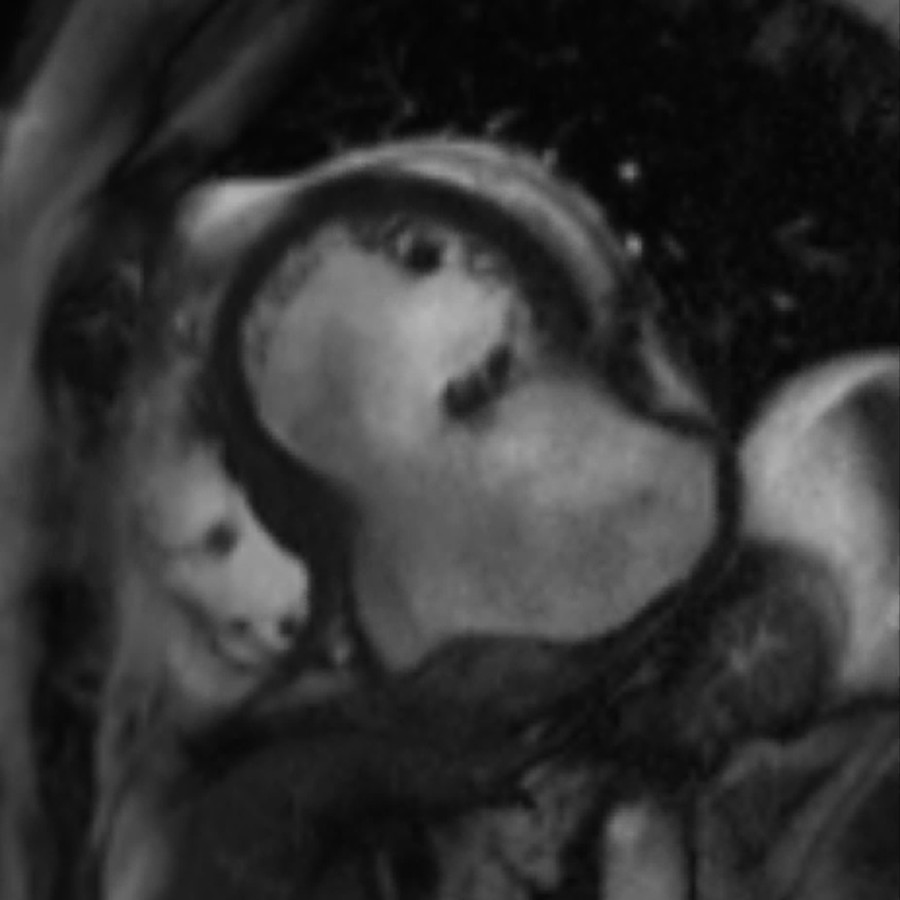
Figure 3.
Cardiac MRI panel. (A) STIR image, two-chamber view. Large bulging aneurysmal sac containing a crescent of thrombus that appears black in these images (arrow). Of note, in the adjacent triangular thrombus (*) which lies in the pericardial space. This indicates that blood has travelled through the myocardium and a free wall rupture has occurred. (B) STIR image, oblique left ventricular outflow tract view. Thrombus can be seen surrounding the inferior wall of the left ventricle and crucially extends beyond the ventricle to the atria (arrows). The thrombus is therefore not contained within the ventricle and again indicates thrombus in the pericardial space and a diagnosis of LVFWR. (C) MAG1 short axis view. The extent of the aneurysm is illustrated with a dotted line that encompasses the usual contour of the left ventricle. The arrow indicates where a disturbance in the left ventricular wall can be visualised as an irregular ‘step-down’. This is the sight of sealed LVFWR. (D) STIR image, short axis view. The same discontinuity in the left ventricular wall can be seen in this image in the same area. In addition, the arrow indicates a thrombus adherent to the site of rupture that is lying in the pericardial space. It is this clot that has sealed the left ventricular free wall and prevented further bleeding. A, atria; V, ventricle; STIR, short τ inversion recovery; LVFWR, left ventricular free wall rupture.
Cine two-chamber view of the left ventricle. The stroke volume of the left ventricle can clearly be seen being offloaded into the large aneurysmal sack visualised inferiorly causing a significant reduction in stroke volume.
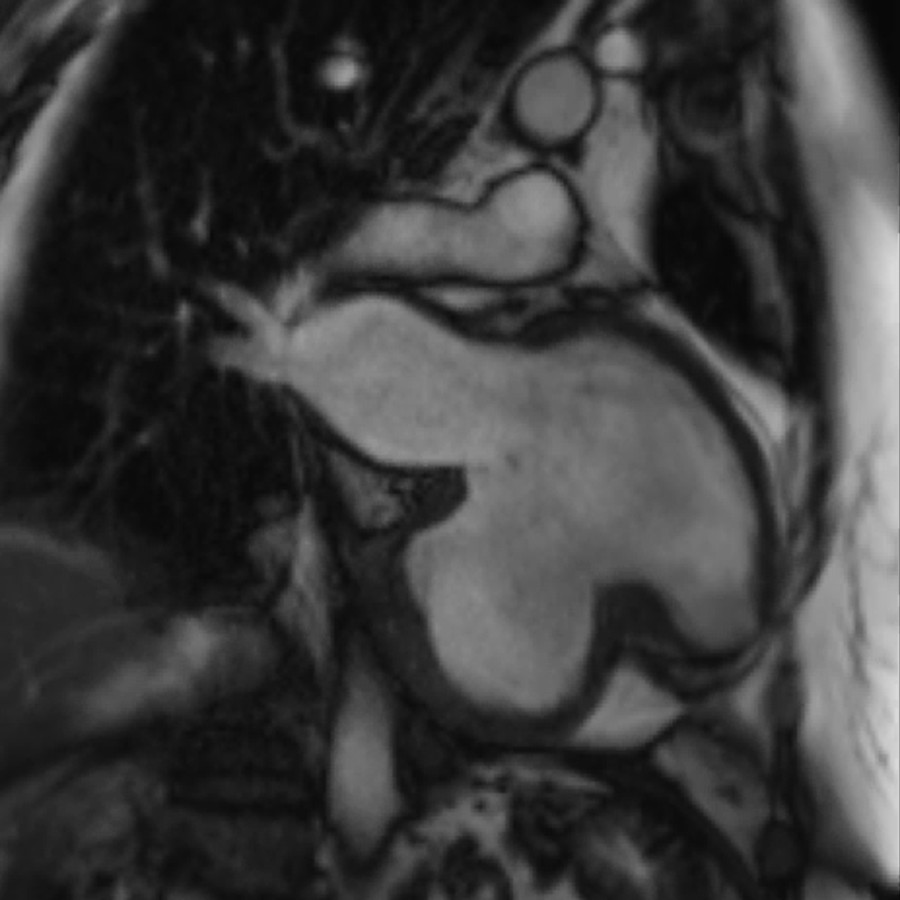
Cine two-chamber view of the left ventricle. Superiorly the left ventricle containing trabeculations can be seen contracting. The inferior chamber is the aneurysmal sac which lacks trabeculations and can be seen clearly expanding to accommodate the left ventricular stroke volume during systole.
Outcome and follow-up
Virtual volume estimation using CMR was crucial in guiding the further management of the patient. The actual EF of the aneurysmal heart was estimated at 24% with a SV of 39 mL/m2. However, virtual volumes estimated using a standard aneurysm repair resection margin indicated a significant improvement in predicted cardiac function. Using virtual volumes the EF improved to 45% with a SV of 44 mL/m2 (table 1). The patient was therefore referred for a surgical opinion to discuss the risks and benefits of pseudoaneurysm resection.
Table 1.
Comparison of actual and virtual volumes based on CMR calculations
| EF (%) | EDV (41–81 mL/m2) | ESV (12–21 mL/m2) | SV (26–56 mL/m2) | |
|---|---|---|---|---|
| Actual volume | 24 | 161 | 122 | 39 |
| Virtual volume | 45 | 98 | 54 | 44 |
Normal reference ranges given in brackets.
Virtual volumes were calculated using a predicted resection margin to assess whether cardiac function would improve post aneurysectomy. Volumes were calculated proportional to an estimated BSA of 1.8 m2.
BSA, body surface area; EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; SV, stroke volume.
On review, 6 months after her CMR, the patient remained stable with optimal medical treatment and was not limited by any cardiac symptoms. Her exercise tolerance allowed her to continue with all of her essential daily activities as well as recreational activities that included regular dance classes. Her follow-up echocardiograms, 6 and 9 months after her CMR, demonstrated unchanged appearances of the pseudoaneurysm with stable moderate left ventricular systolic impairment and mild mitral regurgitation. After a discussion with her family and the surgical team she opted for medical management as she felt that the risks of cardiac surgery outweighed the benefits. She remains stable under regular outpatient follow-up.
Discussion
LVFWR is a rare but grave complication of AMI. It accounts for up to 30% of infarct-related deaths.1
Registry data indicate that FWR is reported in only 0.2% of cases of acute coronary syndrome but occurs 2.9% of AMI complicated by cardiogenic shock.2 3 Mortality rates are improving but are still exceptionally high at 75–80%; although there is a subset of patients who present subacutely, as in our case, who have a better prognosis between 40% and 75%.3 Risk factors include age, female sex, hypertension, lack of, or delayed, reperfusion, lack of preceding angina and patients presenting with their first MI.1
Many patients do not present to medical services as they die suddenly; however, those that do present to hospital tend to present with prolonged anginal chest pain lasting 4–6 h, or shorter episodes of pain over a number of days. Up to a third of patients present subacutely. In such cases, the initial LVFWR becomes contained by means of epicardial/pericardial clot formation and adhesion that seals the LV cavity, preventing complete rupture.1
Echocardiography is clearly invaluable in the acute setting, especially to demonstrate pericardial fluid collection. Awareness of the ability of prompt echocardiography to identify post-MI complications such as pericardial tamponade, ventricular septal rupture and LVFWR is an important learning point. However, echocardiography only has a sensitivity and specificity of 70% and 90%, respectively, for LVFWR.4 Therefore, CMR has an emerging role in the assessment of LVFWR, although it should not be used to delay urgent management. As shown in figure 3, CMR is able to accurately define cardiac anatomy, measure cardiac volume, identify thrombus and identify areas of ischaemic myocardium more accurately than echocardiography.5 6
CMR is also able to effectively differentiate aneurysm from pseudoaneurysm, by examining the location of the aneurysm, the neck-to-body ratio of the aneurysm and the continuity of the myocardial wall. This distinction is essential, as true aneurysm only requires surgical intervention if there are associated symptoms, whereas pseudoaneurysm has a propensity for further rupture so is generally managed surgically. Recent CMR series also suggest that marked delayed gadolinium enhancement of the pericardium is a novel characteristic feature of pseudoaneurysm as opposed to true aneurysm.7
CMR has been used effectively in other cases to identify LVFWR but to our knowledge this is the first published case that used virtual volume calculations to inform management. Other cases include an example where images of an impending rupture were captured hours before the fatal event.8 CT scanning has also been used to detect LV rupture, although both cases went on to have CMRI for definitive evaluation.9 10
Importantly, in the case above, the acute LVFWR was contained with an epicardial thrombus, as demonstrated in figure 3. This lead to the subsequent formation of a left ventricular pseudoaneurysm that remained stable during follow-up echocardiography. The propensity for an untreated pseudoaneurysm to rupture remains high at 30–45%. This, combined with a perioperative mortality of around 10% using modern techniques, means that many patients will opt for surgical management.11
However, there is some evidence suggesting that patients with a left ventricular pseudoaneurysm whom remain haemodynamically stable can be managed medically. Indeed, in a small study of eight patients with postinfarction pseudoaneurysm, there were no mortalities at 5-year follow-up with medical management.12 In addition, in a larger study of 52 patients demonstrated that although patients managed medically had a higher mortality than those managed surgically (60% vs 31%), there were no deaths as a result of pseudoaneurysm rupture at 4-year follow-up in either group. Therefore, it is sensible to take into account the haemodynamic and functional status as well as the comorbidity profile of each individual when deciding on a medical or surgical management strategy, in addition to the wishes of the patient.13 Of note, surgical mortality is significantly higher in patients requiring concomitant mitral valve repair.11
Ultimately, an awareness of LVFWR is essential to identify patients who may warrant urgent surgical intervention. Echocardiography is clearly the investigation of choice in the acute setting; however, CMR offers several unique advantages in those who are stable or presenting subacutely.
Learning points.
Acute cardiac rupture is a grave complication of acute myocardial infarction (AMI) that requires urgent surgical intervention. Therefore, an awareness of symptoms and prompt investigations are key to ensuring optimal outcomes.
Female patients with delayed presentations of their first AMI, delayed reperfusion, without a history of prior angina are most at risk.
Urgent echocardiography is the investigation of choice in those who are acutely unwell.
Up to one-third of patients present subacutely with left ventricular free wall rupture and subsequent pseudoaneurysm formation which has a propensity for further rupture.
Cardiac MR is an emerging imaging modality for the assessment of the haemodynamically stable patient with cardiac rupture. It offers additional information, including estimation of postoperative virtual cardiac volumes, to inform best management strategies.
Footnotes
Contributors: CO wrote the case report and gathered all of the information required for the manuscript. MS provided the CMR images and helped revise the manuscript. MS also provided figure legends for the CMR images.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Raposo L, Andrade MJ, Ferreira J et al. . Subacute left ventricle free wall rupture after acute myocardial infarction: awareness of the clinical signs and early use of echocardiography may be life-saving. Cardiovasc Ultrasound 2006;4:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slater J, Brown RJ, Antonelli TA et al. . Cardiogenic shock due to cardiac free-wall rupture or tamponade after acute myocardial infarction: a report from the SHOCK Trial Registry. Should we emergently revascularize occluded coronaries for cardiogenic shock? J Am Coll Cardiol 2000;36(3 Suppl A):1117–22. [DOI] [PubMed] [Google Scholar]
- 3.Figueras J, Alcalde O, Barrabes JA et al. . Changes in hospital mortality rates in 425 patients with acute ST-elevation myocardial infarction and cardiac rupture over a 30-year period. Circulation 2008;118:2783–9. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Sendon J, Gonzalez A, Lopez de Sa E et al. . Diagnosis of subacute ventricular wall rupture after acute myocardial infarction: sensitivity and specificity of clinical, hemodynamic and echocardiographic criteria. J Am Coll Cardiol 1992;19:1145–53. [DOI] [PubMed] [Google Scholar]
- 5.Krishnan U, McCann GP, Hickey M et al. . Role of contrast-enhanced magnetic resonance imaging in detecting early adverse remodeling and subacute ventricular wall rupture complicating myocardial infarction. Heart Vessels 2008;23:430–2. [DOI] [PubMed] [Google Scholar]
- 6.Dutta R, Kahn AM, Rathod A et al. . Utility of cardiac magnetic resonance imaging in evaluating left ventricular pseudoaneurysm. Congest Heart Fail 2008;14:91–4. [DOI] [PubMed] [Google Scholar]
- 7.Konen E, Merchant N, Gutierrez C et al. . True versus false left ventricular aneurysm: differentiation with MR imaging—initial experience. Radiology 2005;236:65–70. [DOI] [PubMed] [Google Scholar]
- 8.Zoni A, Arisi A, Corradi D et al. . Images in cardiovascular medicine. Magnetic resonance imaging of impending left ventricular rupture after acute myocardial infarction. Circulation 2003;108:498–9. [DOI] [PubMed] [Google Scholar]
- 9.Bohdiewicz PJ. Single photon emission computed tomography radionuclide ventriculography in the noninvasive diagnosis and evaluation of a false left ventricular aneurysm (pseudoaneurysm). Clin Nucl Med 2003;28:821–6. [DOI] [PubMed] [Google Scholar]
- 10.Ohtani H, Katoh H, Saitoh T et al. . Left ventricular pseudo-false aneurysm detected with ECG-gated multidetector computed tomography and cardiac magnetic resonance imaging. Circ J 2010;74:1986–8. [DOI] [PubMed] [Google Scholar]
- 11.Frances C, Romero A, Grady D. Left ventricular pseudoaneurysm. J Am Coll Cardiol 1998;32:557–61. [DOI] [PubMed] [Google Scholar]
- 12.Hung MJ, Cherng WJ, Kuo LT et al. . Echocardiographic characteristics of patients surviving nonsurgically treated left ventricular pseudoaneurysm after acute myocardial infarction. Am J Cardiol 2003;91:328–31. [DOI] [PubMed] [Google Scholar]
- 13.Yeo TC, Malouf JF, Oh JK et al. . Clinical profile and outcome in 52 patients with cardiac pseudoaneurysm. Ann Intern Med 1998;128:299–305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cine two-chamber view of the left ventricle. The stroke volume of the left ventricle can clearly be seen being offloaded into the large aneurysmal sack visualised inferiorly causing a significant reduction in stroke volume.

Cine two-chamber view of the left ventricle. Superiorly the left ventricle containing trabeculations can be seen contracting. The inferior chamber is the aneurysmal sac which lacks trabeculations and can be seen clearly expanding to accommodate the left ventricular stroke volume during systole.