Abstract
Background
The rs10761482 polymorphism of the ANK3 gene has been associated with the occurrence of schizophrenia.
Aim
Assess the relationship between the ANK3 gene and schizophrenia in individuals of Uyghurian descent.
Methods
A total of 630 patients with schizophrenia and 535 healthy controls of Uyghur descent were genotyped for the ANK3 gene rs10761482 locus using Taqman probe technology. SHEsis and SPSS17.0 software were used for data analysis.
Results
There were no significant differences in the genotype or allele frequencies between the case group and control group. Within the case group there was no relationship between gender or age of onset of schizophrenia and the genotype or allele frequencies. Separate analyses among men and among women also failed to identify significant differences in the allele and genotype frequencies between cases and controls or between patients with adolescent-onset schizophrenia and those with adult-onset schizophrenia
Conclusion
Our findings do not support previous reports about the relationship of the ANK3 gene and schizophrenia. In the Uyghur nationality group recruited for this study there was no significant association between the ANK3 gene rs10761482 polymorphism and schizophrenia. If these results are replicated in further studies, then the focus should change to understanding why this widely acknowledged association does not exist in this particular ethnic group.
Keywords: schizophrenia, ANK3 gene, rs10761482 polymorphism, association studies, Uyghur nationality, China
Abstract
背景
ANK3基因rs10761482多态性已被发现与精神 分裂症的发生相关联。
目的
评估新疆维吾尔族人群ANK3基因和精神分裂 症之间的关联。
方法
使用Taqman探针技术对630例新疆维吾尔族 精神分裂症患者和535名新疆维吾尔族健康人群进行 ANK3 基因rs10761482位点的基因分型。采用SHEsis 和SPSS17.0软件进行数据分析。
结果
病例组和对照组之间的基因型和等位基因频率 无显著差异。在病例组,性别或精神分裂症发病年龄 与基因型或等位基因频率之间没有显著关联。将男性 和女性单独分析,病例组与对照组之间的等位基因和 基因型频率均未发现显著差异,青春期发病与成年后 发病的精神分裂症患者之间的等位基因和基因型频率 也无显著差异。
结论
我们的研究结果不支持以往ANK3基因与精神 分裂症有关联的报告。本研究招募的维吾尔族人群中, ANK3 基因rs10761482多态性与精神分裂症之间没有 显著关联。如果这些结果在进一步的研究中得到证实, 那么研究重点将转而了解为什么在这个特定的族群中 不存在上述已经被广泛认可的关联。
1. Background
Although linkage studies suggested that genetic factors play a major role in the onset and course of schizophrenia, the exact mechanisms remain unclear.[1] One gene of particular interest is ANK3 , located on chromosome 10q21. It acts on the early development of the Ranviers nodes of axonsin the central and peripheral nervous systems[2] and, thus, plays a key role in the regulation and differentiation of the nervous system.[3] When the ANK3 gene is knocked out in mice, the mice show abnormal hypothalamic - pituitary - adrenal axis (HPA) functioning.[4] Ankyrin G (ANKG), which is encoded by the ANK3 gene, is crucial for the stability of the neuronal membrane[5],[6] and facilitates the connection between axons and ribbon synapses.[7] ANKG also regulates ion channels involved in the release of neurotransmitters;[8] abnormal expression of ANKG may induce abnormal activity in glutamate receptors (GluRs).[9] Many studies have demonstrated that abnormal release of brain neurotransmitters in different neural pathways is involved in the pathogenesis of schizophrenia. Thus, HPA axis dysfunction and abnormal glutamate activity– both of which are influenced by ANK3 – are key targets in the search for the genetic causes of schizophrenia.
Several studies suggest that ANK3 is important in the pathogenesis of schizophrenia. Athanasiu and colleagues conducted a genome-wide association study (GWAS) in 2663 European individuals with schizophrenia and 13,780 controls and reported that the ANK3 gene rs10761482 polymorphism was associated with schizophrenia.[10] Yuan and colleagues found the same results in a sample of Han Chinese.[11] Another study reported that the ANK3 was also related to the age of onset of schizophrenia.[12] In this study, we aim to assess the relationship of the ANK3 rs10761482 polymorphism with schizophrenia among individuals of Uyghur decent (one of China’s large ethnic minority groups) living in the Xinjiang region of western China and determine whether or not the polymorphism is different in persons with schizophrenia who have an early versus late age of onset.
2. Methods
2.1. Sample
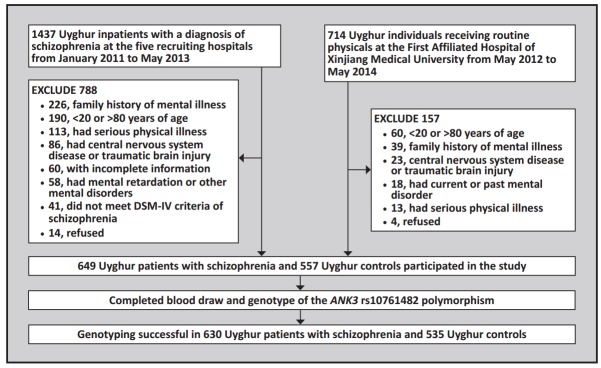
Figure 1 shows the enrollment process for the study. All the participants were natives to Xinjiang of the Uyghur nationality and had no biological connections with each other. Two senior psychiatrists conducted the diagnosis and collected the clinical data in the patient group. We provided a detailed explanation to all participants about the purpose of this study and obtained informed consent signed by the subjects or their guardians. This study was approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University.
Figure 1. Identification of study participants.
2.1.1. Case group
A total of 649 Uyghur inpatients diagnosed with schizophrenia were recruited from six hospitals in the Xinjiang Uyghur Autonomous Region of China between January 2011 and May 2013: the Department of Psychology of the First Affiliated Hospital of Xinjiang Medical University, the Urumqi Peace Hospital, the Hotan Mental Health Hospital, the Kashgar First People’s Hospital, the Yili Mental Health Hospital, and the Aksu Prefecture Kangning Hospital. Inclusion criteria were: (a) 20 to 80 years of age; (b) diagnosis of schizophrenia based on the criteria specified in the Diagnostic and Statistical Manual of Mental Disorders 4th edition (DSMIV) as assessed by two trained psychiatrists using the Chinese version of the Structured Clinical Interview for DSM-IV (SCID)[13] ; (c) no mental retardation or other mental disorders; (d) no family history of mental illnesses; (e) no central nervous system diseases or traumatic brain injury; and (f) no serious physical illnesses, such as cancer, endocrine disease, severe cardiovascular disease, or liver or kidney dysfunction.
The 649 patients in the case group included 389 (60%) males and 260 (40%) females. Their mean (sd) age was 44.4 (12.1) years. Among them, 134 (75 males and 59 females) had the first onset of illness when they were 18 years of age or younger (the ‘adolescent-onset’ group); the remaining 515 (314 males and 201 females) had the first onset after 18 years of age (the ‘adult-onset’ group).
2.1.2. Control group
Individuals of the Uyghur minority who participated in a routine physical examination at the First Affiliated Hospital of Xinjiang Medical University from May 2012 to May 2014 were potential control subjects. They had to meet the same inclusion criteria as the case group (above) except that they had no present or prior history of mental disorders. Potential participants were administered the Composite International Diagnostic Interview (CIDI)by psychology research fellows to identify those with current or past mental illnesses. A total of 557 control subjects participated in the study, including 303 (54%) malesand 254 (46%) females. Their mean age was 45.1 (15.50) years.
There was a borderline excess of males in the case group compared to the control group (60% vs. 54%; χ2=3.76, p=0.052) but there was no significant difference in the mean age of the two groups (t=0.82, p=0.414).
2.2. Genotype assessment
Two milliliters of venous blood was drawn from each participant.Blood samples were prepared for genotyping using the DNA extraction system provided by the Tiangen Biotech Company in Beijing.
Based on findings from previous GWAS studies, we selected the loci rs10761482 of the ANK3 gene for genotyping.According to the PubMed nucleic acid database, the rs10761482 (C/T) is in the first intron of the ANK3 gene in Asian and European populations and the allele frequencies were greater than 0.1.
Taqman probe-based polymerase chain reaction (PCR)was used for genotyping. PCRwas conducted using the GeneAmp PCR 9700 system (Applied Biosystem). Each well of the 384-well plate contains 5μl of PCR reaction material: 40ng DNA, 2.5μl PCR reaction mixture, and 0.035μl Taqman probes.Forty five cycles of amplification were performed using the following settings: 95°C for 10 minutes, 92°C for 15 seconds, and 60°C for 1 min. The product was genotyped using the ABI 7900 DNA detection system (Applied Biosystem, Foster City, CA, USA). In the patient group 97.1% (630/649) of the samples were successfully genotyped; in the control group 96.1% (535/557) were successfully genotyped. In order to ensure accuracy and reliability, we randomly selected 3% of the samples from each of the two groups to genotype again; the same results were obtained from all of these re-tested samples.
2.3. Statistical Analysis
Microsoft Excel was used to construct the database. SPSS17.0 software was used for analysis. Two-sample t-test was used to compare the age of onset and χ2 test was used to compare the male-female ratios. In addition,the SHEsis software (http://analysis.bio-x.cn) was used to test if the data were in accordance with the Hardy-Weinberg equilibrium (HWE) and to analyze differences in the allele frequencies and genotypes between the two groups. Due to the borderline difference in the male-female ratios between the two groups, we also conducted a stratified analysis by gender.
3. Results
3.1. Comparisons of the allele and genotype frequencies between the case group and control group
The distribution of the rs10761482 polymorphisms met the Hardy-Weinberg equilibrium (HWE) requirements in all four subgroups of subjects considered in this analysis: male cases (p=0.059), male controls (p=0.116), female cases (p=0.956), and female controls (p=0.855). No statistically significant differences were found in the rs10761482 allele and genotype frequencies between the 630 cases and 535 controls who were successfully genotyped. As shown in Table 1, similar results were found when the analysis was stratified by gender; neither the allele frequency nor the genotype frequency were significantly different between male cases and controls or between female cases and controls.
Separate comparison of the results of the 381 males with schizophrenia who were successfully genotyped and the 249 females with schizophrenia who were successfully genotyped found no significant difference by gender in the allele frequency (OR=1.01, CI=0.77~1.33, p=0.942) or in the genotype frequency (χ2=1.52, p=0.468).
Table 1.
Comparisons of allele and genotype frequencies of the ANK3gene rs10761482 polymorphism between persons of Uyghur nationality with and without schizophrenia
| rs10761482 | Allele | Genotype | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C n (%) |
T n (%) |
odds ratio |
95%CI | p | C/C n (%) |
C/T n (%) |
T/T n (%) |
χ2 | p | |
| BOTH GENDERS | ||||||||||
| cases (n=630) | 983 (78.0) | 277 (22.0) | 1.00 | [0.83~1.22] | 0.967 | 377 (59.8) | 229 (36.3) | 24 (3.8) | 0.01 | 0.995 |
| controls (n=535) | 834 (77.9) | 236 (22.1) | 320 (59.8) | 194 (36.3) | 21 (3.9) | |||||
| MALES | ||||||||||
| cases (n=381) | 595 (78.1) | 167 (21.9) | 0.98 | [0.75~1.27] | 0.885 | 226 (59.3) | 143 (37.5) | 12 (3.1) | 0.04 | 0.981 |
| controls (n=293) | 460 (78.5) | 126 (21.5) | 176 (60.1) | 108 (36.9) | 9 (3.1) | |||||
| FEMALES | ||||||||||
| cases (n=249) | 388 (77.9) | 110 (22.1) | 1.04 | [0.77~1.40] | 0.810 | 151 (60.6) | 86 (34.5) | 12 (4.8) | 0.074 | 0.967 |
| controls (n=242) | 374 (77.3) | 110 (22.7) | 144 (59.5) | 86 (35.5) | 12 (5.0) | |||||
CI, confidence interval
3.2. Association between the onset age and the ANK3 gene rs10761482 polymorphism
As shown in Table 2, there were no differences in either the genotype or the allele frequencies of the ANK3 rs10761482 polymorphism between the 131 individuals with adolescent-onset schizophrenia and the 499 individuals with adult-onset schizophrenia who successfully completed the genotyping. Stratified analysis revealed similar results by gender: the frequencies of alleles and genotypes between male adolescent-onset and adult-onset patients with schizophrenia were not significantly different, and the frequencies of alleles and genotypes between female adolescent-onset and adult-onset patients were also not significantly different.
Table 2.
Comparisons of allele and genotype frequencies of the ANK3 gene rs10761482 polymorphism between persons of Uyghur nationality with adolescent-onset schizophrenia (ADL) and persons of Uyghur nationality with adult-onset schizophrenia (ADU)
| rs10761482 | Allele | Genotype | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C n (%) |
T n (%) |
odds ratio |
95%CI | p | C/C n (%) |
C/T n (%) |
T/T n (%) |
χ2 | p | |
| BOTH GENDERS | ||||||||||
| ADL group (n=131) | 203 (77.5) | 59 (22.5) | 0.96 | [0.69~1.33] | 0.814 | 77 (58.8) | 49 (37.4) | 5 (3.8) | 0.08 | 0.960 |
| ADU group (n=499) | 780 (78.2) | 218 (21.8) | 300 (60.1) | 180 (36.1) | 19 (3.8) | |||||
| MALES | ||||||||||
| ADL group (n=74) | 113 (76.4) | 35 (23.6) | 0.88 | [0.80~1.35] | 0.570 | 43 (58.1) | 27 (36.5) | 4 (5.4) | 1.53 | 0.465 |
| ADU group (n=307) | 482 (78.5) | 132 (21.5) | 183 (59.6) | 116 (37.8) | 8 (2.6) | |||||
| FEMALES | ||||||||||
| ADL group (n=57) | 90 (78.9) | 24 (21.1) | 1.08 | [0.65~1.80] | 0.761 | 34 (59.6) | 22 (38.6) | 1 (1.8) | 1.181 | 0.405 |
| ADU group (n=192) | 298 (77.6) | 86 (22.4) | 117 (60.9) | 64 (33.3) | 11 (5.7) | |||||
CI, confidence interval
Separate analyses comparing the 131 adolescentonset patients’ results to those of all 535 controls found no difference in allele frequency (OR=0.97, CI=0.59~1.45, p=0.871) or in genotype frequency (χ2 =0.06, p=0.971). A similar comparison of the 499 adult-onset patients’ results to those of all controls found no difference in allele frequency (OR=1.01, CI=0.82~1.28, p=0.907) or in genotype frequency (χ2 =0.02, p=0.992). These comparisons remained statistically insignificant after stratifying by gender (results provided on request).
4. Discussion
4.1. Main findings
This study is the first to explore the role of the ANK3 gene in schizophrenia in a relatively large sample of individuals of Uyghur descent. We found no statistically significant differences between cases and controls in either the allele frequencies or the genotypes of one of the ANK3 polymorphisms that is most frequently associated with schizophrenia. We also found no link between the ANK3 rs10761482 polymorphism and the age of onset of schizophrenia. Other studies[14] have also failed to replicate findings about the role of ANK3 in schizophrenia, but the weight of the evidence – including a meta-analysis[11] confirming the role of allele C of the ANK3 rs10761482 polymorphism and of allele T of the rs10994336 locus – still supports the hypothesized role of ANK3 in the pathogenesis of schizophrenia. Several studies have identified mechanisms via which ANK3 could be involved; for example, allele C of the ANK3 rs9804190 polymorphism is associated with the down-regulation of mRNA expression in schizophrenia.[15] Nevertheless, our negative findings and those of other investigators suggest that other factors may play a role in moderating the effects of ANK3 .
4.2. Limitations
Several factors may have contributed to our null findings. (a) The study sample was limited to individuals of the Uyghur minority group in China, a fairly homogenous group (with limited out-group marriage) that has both European and Asian genetic characteristics. Despite the similar C allele frequency of the locus rs10761482 in our sample (78%) to that reported for the European and Asian populations (74.8% and 75%, respectively, based on the PubMed database), the Uyghur group may have unique genetic characteristics that alter the relationship between ANK3 and schizophrenia. (b) In limiting our sample to individuals with schizophrenia who do not have a family history of mental illness, we may have decreased the relative importance of genetic factors in the group of patients considered in the analysis. (c) We did not assess several other ANK3 loci that may be related to the pathogenesis of schizophrenia including rs10761482, rs9804190, and rs10994336.[11],[15] (d) Some studies suggest that genetic polymorphisms are associated with specific clinical subtypes of schizophrenia;[16],[17] other than considering age of onset, we did not subclassify our results by other potentially important factors. (e) The sample size was too small to justify stratifying results by multiple variables.
4.3. Implications
Replication of widely accepted genetic findings about psychiatric illnesses in different, relatively homogenous ethnic groups may help identify factors that moderate the role of these presumed ‘universal’ genetic factors. Our failure to replicate findings of a relationship between the ANK3 gene rs10761482 polymorphism and schizophrenia in a relatively large sample of patients with schizophrenia from the Uyghur minority group in western China is an example of the potential promise of such studies. If these results are replicated in further studies, then the focus should change to understanding why this widely acknowledged association does not exist in this particular ethnic group. Such follow-up studies would have considerable value in highlighting the genetic environment in which ANK3 does or does not exert a role in the pathogenesis of schizophrenia.
Acknowledgments
Acknowledgement: The authors thank the students in the School of Public Health at Ningxia Medical University and staff members of the Ningxia Center for Disease Control and Prevention for their assistance in the conduct of the study.
Biography

Xianjiang Zhong obtained his Master’s degree from Xinjiang Medical University in June 2014. He has been a resident physician in the Mental Health Department of the First People’s Hospital in Xiantao City, Hubei Province since July 2014. His research interest is in the molecular genetics of schizophrenia.
Funding Statement
This work was supported by the Natural Science Foundation of China (project name: ‘de novo CNV in schizophrenia in persons of Uyghur nationality in Xinjiang‘, no. 81360209) and the Xinjiang Uyghur Autonomous Region Science and Technology Fund (project name:’Establishing a genetic database for depression in persons of Uyghur nationality in Xinjiang’, no. 2010211A51).
Conflict of interest: The authors report no conflicts of interests related to this study.
Ethics approval: This study was approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University.
Informed consent: All participants or their guardians provided written informed consent to participate in the study.
References
- 1.Farmer AE, McGuffin P. The pathogenesis and management of schizophrenia. Drugs. 2088;35:177–185. doi: 10.2165/00003495-198835020-00006. [DOI] [PubMed] [Google Scholar]
- 2.Kapfhamer D, Miller DE, Lambert S, Bennett V, Glover TW, Burmeister M. Chromosomal localization of the ankyrinG gene (ANK3/Ank3) to human 10q21 and mouse 10. Genomics. 1995;27(1):189–191. doi: 10.1006/geno.1995.1023. [DOI] [PubMed] [Google Scholar]
- 3.Hansell NK, Medland SE, Ferreira MAR, Geffen GM, Zhu G, Montgomery GW, et al. Linkage analyses of event-related potential slow wave phenotypes recorded in a working memory task. Behav Genet. 2006;36(1):29–44. doi: 10.1007/s10519-005-9002-2. [DOI] [PubMed] [Google Scholar]
- 4.Sobotzik JM, Sie JM, Politi C, Del Turco D, Bennett V, Deller T, et al. AnkyrinG is required to maintain axo-dendritic polarity in vivo. Proc Natl Acad Sci USA. 2009;104(41):17564–17569. doi: 10.1073/pnas.0909267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett V. Ankyrins: adaptors between diverse plasma membrane proteins and the cytoplasm. J Biol Chem. 1992;267(13):8703–8706. [PubMed] [Google Scholar]
- 6.Bennett V, Otto E, Kunimoto M, Kordeli E, Lambert S. Diversity of ankyrins in the brain. Biochem Soc Trans. 1991;19(4):1034–1039. doi: 10.1042/bst0191034. [DOI] [PubMed] [Google Scholar]
- 7.Hedstrom KL, Ogawa Y, Rasband MN. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. Cell Biol. 2008;183(4):635–640. doi: 10.1083/jcb.200806112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kosaka T, Komada M, Kosaka K. Sodium channel cluster, betaIV-spectrin and ankyrinG positive ‘‘hot spots’’ on dendritic segments of parvalbumin-containing neurons and some other neurons in the mouse and rat main olfactory bulbs. Neurosci Res. 2008;62(3):176–186. doi: 10.1016/j.neures.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Talkowski ME, Rosenfeld JA, Blumenthal I, Pillalamarri V, Chiang C, Heilbut A, et al. Sequencing chromosomal abnormalities reveals neurodevelopmental loci that confer risk across diagnostic boundaries. Cell. 2012;149(3):525–537. doi: 10.1016/j.cell.2012.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Athanasiu L, Mattingsdal M, Kähler AK, Brown A, Gustafsson O, Agartz I, et al. Gene variants associated with schizophrenia in a Norwegian genome-wide study are replicated in a large European cohort. J Psychiatr Res. 2012;44(12):748–753. doi: 10.1016/j.jpsychires.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan A, Yi Z, Wang Q, Sun J, Li Z, Du Y, et al. ANK3 as a risk gene for schizophrenia: new data in Han Chinese and meta analysis. Am J Med Genet B Neuropsychiatr Genet. 2012;159(8):997–1005. doi: 10.1002/ajmg.b.32112. [DOI] [PubMed] [Google Scholar]
- 12.Talkowski ME, Rosenfeld JA, Blumenthal I, Pillalamarri V, Chiang C, Heilbut A, et al. Sequencing choromosomal abnormalities reveals neurodevelopmental loci that confer risk across diagnostic boundaries. Cell. 2012;149(3):525–537. doi: 10.1016/j.cell.2012.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips MR, Liu XH. Adapted Chinese version of Structured Clinical Interview for DSM-IV-TR Axis I Disorders,Research Version, Patient Edition (SCID-I/P) by Michael B. First, Robert L. Spitzer, Miriam Gibbon, and Janet B.W. Williams. Shanghai: Suicide Research and Prevention Center, Shanghai Mental Health Center; 2011. [Google Scholar]
- 14.Gella A, Segura M, Durany N, Pfuhlmann B, Stöber G, Gawlik M. Is Ankyrin a genetic risk factor for psychiatric phenotypes? BMC Psychiatry. 2011;11(103):1–5. doi: 10.1186/1471-244X-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roussos P, Katsel P, Davis KL, Bitsios P, Giakoumaki SG, Jogia J, et al. Molecular and genetic evidence for abnormalities in the nodes of Ranvier in schizophrenia. Arch Gen Psychiatry. 2012;69(1):7–15. doi: 10.1001/archgenpsychiatry.2011.110. [DOI] [PubMed] [Google Scholar]
- 16.Su L, Wei B, Chen Q, Feng Q, Pan R, Zhou Y, et al. [Association study of PRODH gene variant rs385440 with schizophrenia in Zhuang and Han nationality of Guangxi] Zhonghua Xing Wei Yi Xue Yu Nao Ke Xue Za Zhi. 2012;21(1):36–39. Chinese. [Google Scholar]
- 17.Guo W, Li W, Zhang H, Hao W. [Association study of disrupted-in-schizophrenia-1 gene single nucleotide polymorphism with schizophrenia in Han Chinese population] Zhonghua Xing Wei Yi Xue Yu Nao Ke Xue Za Zhi. 2012;21(4):337–339. Chinese. [Google Scholar]


