Abstract
Skin biopsies are usually undertaken to confirm a clinical diagnosis, to remove a lesion, and to determine the adequacy of excised tissue margin. A surgical margin is technically defined as the “edge” of the tissue removed. The term is especially pertinent when the tissue excised is suspected of being involved by a malignant process. One of the most important predictive and prognostic factors of a malignant lesion is whether the margins of the resected specimen are involved by the tumor or not. The purpose of this review is to provide an insight into grossing of a skin biopsy specimen with emphasis on techniques and reporting of excision biopsy margins.
Keywords: Inking, margins, skin excision biopsy
Introduction
What was known?
The knowledge whether a resected specimen is free of tumour at the margins is one of the important goals of reporting. The use of India ink to identify margins is an old technique in surgical pathology grossing, with the limitation that only one margin can be identified.
Grossing is defined as examination and dissection of surgical specimens along with preparation of section from the tissue requiring microscopic examination.[1] One of the most relevant goals of a surgical excision is to determine the status of the margins and hence the adequacy of the surgical excision. In a malignancy, the further management understandably depends on whether the excision margins are clear or not. Thus the accurate and timely reporting on the status of margins is of utmost importance.[2]
It is proved beyond ambiguity that the chances of local recurrence are much more when the margins are positive (involved).[3,4] The status of the margins can be determined by frozen section, on cytology or on routine histopathology.[5] This article re-emphasizes the importance of precise sampling and accurate reporting of margins in practice of dermatopathology. This review also provides guideline to carry out the grossing procedure especially using multicolored inks.
General principles of grossing
Complete information data regarding the demographic and clinical profile of the case should be provided in the requisition form including the details of any previous biopsies undertaken.[6,7] The diagnostic material should be transferred rapidly to the laboratory in an appropriate fixative. Larger specimens are ideally grossed in the fresh state while the small biopsies after an overnight fixation.[8] Biopsies from different sites should be sent in different containers and if not feasible the various sites should be marked in different color sutures or ink.[9] Once the laboratory personnel are sure that the specimen and the request form correspond, the specimen dimension, color, shape, and weight are recorded as per the standard protocol.[3,9] A detailed description of the lesion is noted and a gross photograph taken. If use of ancillary techniques is anticipated the tissue should be handled accordingly.[6] The pathologist grosses the specimen, sampling the margins [radial/tangential] first followed by the main lesion. Smaller specimens are submitted in entirety for examination. The specimens are arbitrarily classified as small (<2 cm), regular (2-5 cm), large (5-10 cm), and extra large (>10 cm).[10]
Concept of margins
A margin is said to be positive when the tumor cells are seen at the inked margin and negative when they are absent or present away from the “inked margin.”[2] Another vague term used frequently by the pathologists is the “close margin” which implies that tumor cells are lying in the vicinity of excised margin (varies anywhere between 1mm and 5 mm). However, in this era of personalized cancer therapy and frequent use of systemic agents the concept of “close” margin has lost its relevance. The pathologist should either report the margin as positive or give precise distance of the malignant cells from inked margin. This paradigm shift was brought out in the article by Anne Paxton, where it was shown that re-excisions in cases of close margin were mostly unnecessary and did not accomplish much except adding to patient morbidity.[2]
Most malignant diseases have two components, a loco-regional component and a metastatic component. The local disease is taken care of by excision or debulking of the lesion. The metastatic component is tackled most often by chemotherapy or radiation.[11] When a clear margin is obtained, one can be reasonably sure that the chances of local disease recurrence would be much lesser. To avoid resurgery-associated issues, many surgeons prefer to remove a lesion with larger chunk of tissue than required. A paradigm shift in this approach to surgery is that “bigger is not better.” A bigger tissue removal does not provide any added advantage in local disease control. Another, removing larger chunks of tissue, is that it gives bad reconstructive results.[12] In this light the pathologist needs to standardize the process of accurate grossing an reporting of excise margins.
Types of skin biopsies
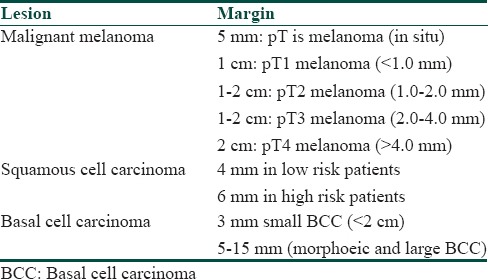
Specimens of skin received for pathological examination could of the following types: excision biopsy, incision biopsy, shave/tangential biopsy, punch biopsy or curetting.[13] The epidermis, dermis and/or subcutaneous tissue should be included in the biopsy. A fine-needle aspiration sample is also occasionally sent. To elaborate, an excision is a completely cut-out lesion for diagnosis and treatment while an incisional biopsy is a segment of the lesion removed for diagnosis only. A punch biopsy is 2-6 mm of tissue removed for diagnostic purpose. A curette consists of fragments of tissue removed using a spoon-shaped bone curette primarily for treatment. A shave biopsy is a horizontal section of the skin, removed either for diagnosis or treatment.[14] The recommended margins for various malignant lesions of skin are different. In melanoma optimal excision margins (from the edge of the melanoma lesion) suggested are as follows: for In situ melanomas, melanomas of thickness <1 mm, melanomas of thickness 1-4 mm, and >4 mm deep melanomas the margin are supposed to be 5 mm, 1 cm, 2 cm, and 2-5 cm, respectively.[15,16] The suggested margin to be taken in squamous and basal cell carcinomas are given in Table 1.[17,18]
Table 1.
Recommended excision margins in skin tumors
Types of margins
The two widely used techniques of sampling margins are:
Shaved margins (en-face, tangential margin): Here a snippet of tissue is taken parallel to the plane of resection. It is used when a tubular structure is being grossed (oesophagus, colon, etc.) or when the tumor is far away from the resected ends (>2 cm). This method has the advantage of sampling more quantity of tissue
Radial margins: when the margin grossly appears close to the tumor (<2 cm) and an anatomical relationship between the margin and the tumor is to be seen; radial margins are helpful. The disadvantage here is limited amount of tissue sampling.
Handling of margins
The laboratory usually gets the specimen with two labeled margins. The specimen is painted on the anterior, posterior, lateral, medial, superior, and inferior aspect with multicolored ink.[9,19,20] The coloring can be done either using a painting brush or a disposable tooth pick. For bigger specimens a thick brush is used and for inking delicate tissue a finer brush is suggested. Following this step it is important to dry the ink completely, either by air drying (5-10 min) or using a hair dryer. The latter is used especially when the weather is humid. After this the tissue is bread-loafed and put in adequate quantity of formalin for fixing and regular processing.[21] It is suggested that every laboratory standardize the colors used to paint a particular margin so that the reporting pathologist recognizes the margin being seen Figure 1a.
Figure 1.
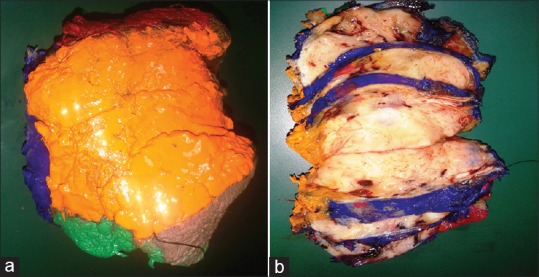
(a) Surfaces of skin tumor painted in multicolored inks (b) Bread loafing of the speciemen depicting the extent of the lesion
After fixation serial sections are taken in a “slice-bread pattern.” Every 2-3 mm should be sampled [Figure 1b] In case when lesion is not grossly visible submit the entire specimens. A specimen <10 mm should also be grossed and submitted in entirety. When the case is of squamous cell carcinoma or of basal cell carcinoma the ulcerated areas and areas of maximum thickness should be sampled cautiously.[15]
Material used for inking
The options available for inking are India ink, gelatines, commercially prepared tissue maker systems, acrylic paints. The advantages and disadvantages of these agents are discussed in Table 2.
Table 2.
Comparison of different coloring agents in histopathology

The AJCP recommends that all the margins need to be marked before grossing, preferably by the operating surgeon.[12,13,14] Multiple colors allow identification of five surgical margins rather than just three, two short axis margins, the two long axis margins, and the deep margin. Evaluation of the five surgical margins can identify the specific site of an existing tumor and its probable extent beyond the surgical margin of resection. This also gives a superior orientation of specimen compared to traditional suture marking methods. The postgrossing reconstruction of the specimen is also easier when the surfaces are marked unambiguously in various colored inks.
Whatever the method used, the inking should be permanent, should not interfere with the tissue processing and staining procedures and should be cost-effective [Figure 2a and b] The material chosen should not be messy or seep in adjacent areas. It is preferable that the ink show up on immunohistochemical procedures.[21,22]
Figure 2.
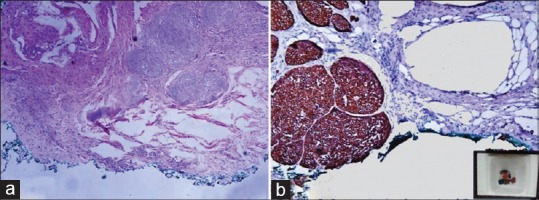
(a) Microphotograph of the basal cell carcinoma. The base painted in green color is free of tumor ×100, H and E (b) Microphotograph of the basal cell carcinoma, immunopositive for pancytokeratin. The green ink is seen on IHC section. The inset shows paraffin block containing multiple pieces of tissue marked in different colors, for identification ×200, IHC
We have used the faithful India ink in our laboratory for long. It is a very reliable, hardy, and inexpensive dye. The problem with this coloring agent is that in a given specimen only one surface can be marked. The use of acrylic painting colors have been recently tested and found to be an excellent method as the various surfaces can be marked in different colors and is also cost-effective.[22]
The acrylic paints in our laboratory are found to be of excellent result using some particular shades of color (black, blue, and green), while some colors did not give a very crisp delineation (red, orange, and mauve). Colors like yellow were found unsuitable for use [unpublished data]. Multiple margins were processed in one block without any color losing its crispness. When these blocks were used for immunohistochemical staining the color withstood the procedure and showed up brightly on microscopy.[23,24,25] In our experience when we ran immunohistochemical stains on sections marked by acrylic colors the results were equally good. The whole process in long run proved to be more economical and less time consuming. This indigenous method worked equally well compared to the more expensive commercially available inks.
Approach to adnexal tumors
When the excision specimen is of an adnexal tumor the anatomic landmarks, relation of the tumor to the overlying skin, blood vessels, and nerves, and weather the underlying bone and muscle are involved, is to be assessed. The dimensions are to be noted and the cut surface should be looked for circumscription, necrosis, fibrosis, or myxoid change. The lymph nodes should be searched for and submitted. If the adnexal tumor is small the margins should be painted in different colors and grossed accordingly. It is always better to take the margin along with the tumor (a radial margin) as the orientation is easy to understand. If the tumor is far away then shaved margins are the preferable option. The latter is specially pertinent to morphoeic forms of BCC which has higher tendency of recurrence and excised with a margin as large as 15 mm.[18]
Conclusion
A correct and unambiguous report regarding the surgical margins of an excised malignant specimen has an immense bearing on the final management of the case. The use of multicolored dyes to mark margins makes assessment process more accurate. This is also a cost-effective and time-saving measure to increase the efficiency in the laboratory.
What is new?
The margins of the resected specimen these days are being painted in the operation theatre itself by the surgical team eliminating the use of traditional orientation sutures. When cost is not a constraint commercial multicolored inks are being used. Acrylic colors provide an economical and sturdy alternative to expensive marking inks.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Romaguer R, Nassiri M, Morales AR. Tools to facilitate and standardize grossing. Histologic. 2003;36:17–21. [Google Scholar]
- 2.Paxton A. On lumpectomy surgical margins, a push for clarity. [Last accessed on 2013 Jul 16]. Available from: http://www.cap.org/., http://www.cap.org/apps/cap.portal .
- 3.Taghian A, Mohiuddin M, Jagsi R, Goldberg S, Ceilley E, Powell S. Current perceptions regarding surgical margin status after breast-conserving therapy: Results of a survey. Ann Surg. 2005;241:629–39. doi: 10.1097/01.sla.0000157272.04803.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singletary SE. Surgical margins in patients with early-stage breast cancer treated with breast conservation therapy. Am J Surg. 2002;184:383–93. doi: 10.1016/s0002-9610(02)01012-7. [DOI] [PubMed] [Google Scholar]
- 5.Ranchod M. Intraoperative consultations in surgical pathology. In: Weider N, Cote RJ, Suster S, Weiss LM, editors. Modern Surgical Pathology. 1st ed. Philadelphia: WB Saunders; 2003. pp. 17–34. [Google Scholar]
- 6.Juan Rosai, Ackerman LV. 9th ed. Vol. 1. St. Louis, Missouri: Mosby, Elsevier; 2004. Rosai and Ackerman's Surgical Pathology; p. 25. [Google Scholar]
- 7.Oliver RJ, Sloan P, Pemberton MN. Oral biopsies: Methods and applications. Br Dent J. 2004;196:329–33. doi: 10.1038/sj.bdj.4811075. quiz 362. [DOI] [PubMed] [Google Scholar]
- 8.Paul E, Billing, William E, Grizzle . The gross room/surgical cut up. In: John D, Bancroft, Marilyn Gamble, editors. Theory and practice of histological techniques. 6th ed. Churchill Livingstone: Elsevier; 2008. pp. 75–82. [Google Scholar]
- 9.Rao RS, Premalatha BR. Grossing in oral pathology: General principles and guidelines. World J Dent. 2010;1:35–41. [Google Scholar]
- 10.Izak B. Dimenstein, grossing techniques in surgical pathology. [Last accessed on 2013 Jul 15]. Available from: http://H:/skin%20margin%20articles/Skin%20Excision%20GROSSING%20TECHNOLOGY%20in%20SURGICAL%20PATHOLOGY.htm .
- 11.McCahill LE, Single RM, Aiello Bowles EJ, Feigelson HS, James TA, Barney T, et al. Variability in reexcision following breast conservation surgery. JAMA. 2012;307:467–75. doi: 10.1001/jama.2012.43. [DOI] [PubMed] [Google Scholar]
- 12.Freedman G, Fowble B, Hanlon A, Nicolaou N, Fein D, Hoffman J, et al. Patients with early stage invasive cancer with close or positive margins treated with conservative surgery and radiation have an increased risk of breast recurrence that is delayed by adjuvant systemic therapy. Int J Radiat Oncol Biol Phys. 1999;44:1005–15. doi: 10.1016/s0360-3016(99)00112-1. [DOI] [PubMed] [Google Scholar]
- 13.Morrow M, Harris JR, Schnitt SJ. Surgical margins in lumpectomy for breast cancer: Bigger is not better. N Engl J Med. 2012;367:79–82. doi: 10.1056/NEJMsb1202521. [DOI] [PubMed] [Google Scholar]
- 14.Ray S. Gross examination of skin specimens. [Last accessed on 2013 Jul 13]. Available from: http://www.histopathology.india.net/dermatopathology.Htm .
- 15.Amanda Oakley. Processing skin biopsies. 2013. Jul 10, [Last accessed on 2013 Jul 10]. Retreived on. Available from: http://www.dermnetnz.org/doctors/lesions/biopsy.html .
- 16.ELEC, Optimal excision margins for primary cutaneous melanoma. National Guideline Clearinghouse (NGC),Rockville, Maryland. Agency for Healthcare Research and Quality (AHRQ) [Last accessed 2013 Jul 17]. Available from: http://www.guideline. gov/content.aspx?id=34597 .
- 17.Brodland DG, Zitelli JA. Surgical margins for excision of primary cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1992;27:241–8. doi: 10.1016/0190-9622(92)70178-i. [DOI] [PubMed] [Google Scholar]
- 18.Telfer NR, Colver GB, Morton CA. British Association of Dermatologists. Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159:35–48. doi: 10.1111/j.1365-2133.2008.08666.x. [DOI] [PubMed] [Google Scholar]
- 19.Lens MB, Nathan P, Bataille V. Excision margins for primary cutaneous melanoma: Updated pooled analysis of randomized controlled trials. Arch Surg. 2007;142:885–93. doi: 10.1001/archsurg.142.9.885. [DOI] [PubMed] [Google Scholar]
- 20.Dimenstein IB. Grossing biopsies: An introduction to general principles and techniques. Ann Diagn Pathol. 2009;13:106–13. doi: 10.1016/j.anndiagpath.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Pedran A. Breast. In: Westra WH, Hruban RH, Phelps TH, Isacson C, editors. Surgical Pathology Dissection. An Illustrated Guide. 2nd ed. New York: Springer-Verlag; 2003. pp. 132–41. [Google Scholar]
- 22.Tampi C. In search of the rainbow: Colored inks in surgical pathology. Indian J Pathol Microbiol. 2012;55:154–7. doi: 10.4103/0377-4929.97843. [DOI] [PubMed] [Google Scholar]
- 23.Shinde V, Phelan C, Gater W, Thomas J. Inking a specimen without the mess. J Clin Pathol. 2008;61:783. doi: 10.1136/jcp.2008.055962. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong JS, Weinzwieg IP, Davies JD. Differential marking of excision planes in screened breast lesions by organically coloured gelatins. J Clin Pathol. 1990;43:604–7. doi: 10.1136/jcp.43.7.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng MV, Koppie TM, Duh QY, Stoller ML. Novel method of assessing surgical margin status in laparoscopic specimens. Urology. 2001;58:677–81. doi: 10.1016/s0090-4295(01)01417-0. [DOI] [PubMed] [Google Scholar]


