FIG 2.
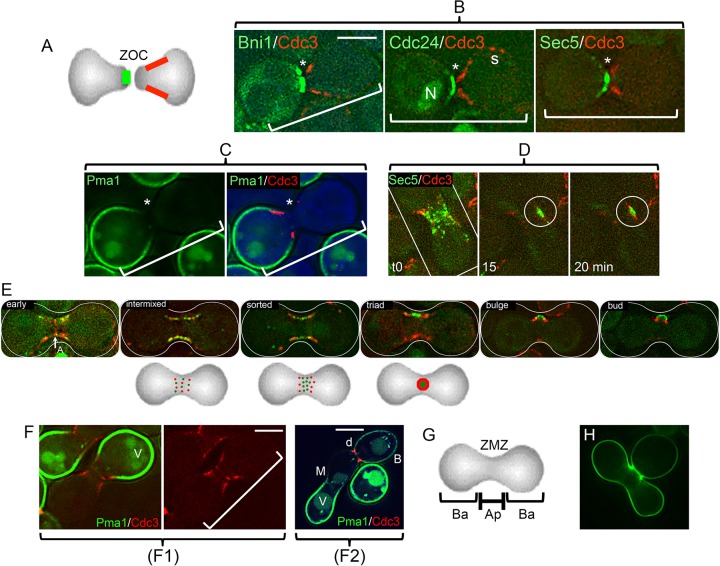
Initial bud formation and reorganization of proteins from the ZOC. (A) Diagram of typical apical and basal protein distributions. The two opposed cells have established a ZOC. They have not yet fused. Selected cortical proteins assume highly polarized distributions at this point. Two distributions are indicated: apical (green) and a subapical “collar” (red). Proteins implicated in membrane fusion, actin polarization, and secretion are in the apical group (panel B; see Fig. S2A in the supplemental material). Septins form the collar. A further group has a basal orientation (see below). (B) Examples of distributions of GFP-tagged proteins that are adjacent to the ZOC (*). In each case, a cell expressing the tagged protein in question (the formin Bni1; the guanine exchange factor for Cdc42, Cdc24; or the exocyst protein Sec5) was crossed with a cell expressing a septin (Cdc3-mCherry). Note that the septin forms a collar subapical to the ZOC (35). The example for Cdc24-GFP—some of which is in the nucleus (N)—shows that septins can also persist at cortical sites (s) that mark sites of previous cytokinesis. The brackets mark the position and orientation of the apposed pairs of cells. The strains were ATY5176 (Bni1), ATY4980 (Cdc24), ATY4312 (Sec5), and ATY5545 (Cdc3). (See Fig. S1A in the supplemental material.) (C) Example of a protein that has a basal distribution. A cell expressing both the GFP-tagged plasma membrane proton ATPase, Pma1, and the septin, Cdc3-mCherry, was crossed with a nonfluorescent cell. The left image shows only GFP. The right image shows both signals, as well as a bright-field image in blue. The strains were ATY5297 × ATY5545. (D) Scattering of an exocyst protein along the ZMZ cortex. Time lapse series of a cell expressing Sec5-GFP crossed with a cell expressing Cdc3-mCherry. Note that at time zero many foci of Sec5-GFP are beginning to be flanked by the septin. At the 15-min time point, Sec5-GFP forms a patch (circled) before completion of the septin ring to form a triad at 20 min. The strains were ATY4312 × ATY5545. (E) Stages of redistribution of the apical protein, Cdc24-GFP, and Cdc3-mCherry. After cell fusion, the nuclear pool of Cdc24-GFP vanishes, the septin annulus (A) is transiently visible, and Cdc3-mCherry and Cdc24-GFP resolve into foci that then scatter and intermix throughout the cortex of the ZMZ. Over the following 10 to 20 min, they sort so that the red foci (septin) progressively flank the green signal. This culminates in the formation of a characteristic “triad,” which subsequently bends and can be recognized as a small bud, which will further enlarge. The three diagrams beneath the micrographs illustrate the progressive morphogenesis that gives rise to the triad. To avoid photobleaching, a composite, not time lapse, series is shown. The strains were ATY4980 × ATY5545. (F) Domain separation persists after cell fusion. (F1) A cell expressing Pma1-GFP and Cdc3-mCherry was crossed with an unlabeled cell, and the two cells have fused. The red septin signal has redistributed symmetrically and shows initial stages of formation of the medial annulus. Note that septins continue to abut on Pma1-GFP, which does not invade the ZMZ. For clarity, the right-hand image includes only the red. V, vacuole. The strains were ATY5896 × ATY4373. (F2) Crosses were conducted between cells expressing Pma1-GFP and cells expressing Cdc3-mCherry. After 5 h, note the sharp discontinuity (d) of distribution of Pma1-GFP at the bud neck. Also note the lack of transfer beyond the ZMZ (M) of preexisting Pma1-GFP from the parent that contributed the lower-left portion of the zygote. B, bud. The strains were ATY5297 × ATY5545. (G) Diagram of apical (Ap) and basal (Ba) domains of early zygotes. The ZMZ is labeled. (H) Overview of the initial bud. Note the size of the nearly mature medial bud of this calcofluor white-stained zygote. It is not unusual to encounter initial buds that are even larger relative to the size of the body of the zygote. The calcofluor white signal is pseudocolored green. The strains were ATY3852 × ATY4373. Scale bars, 5 μm.