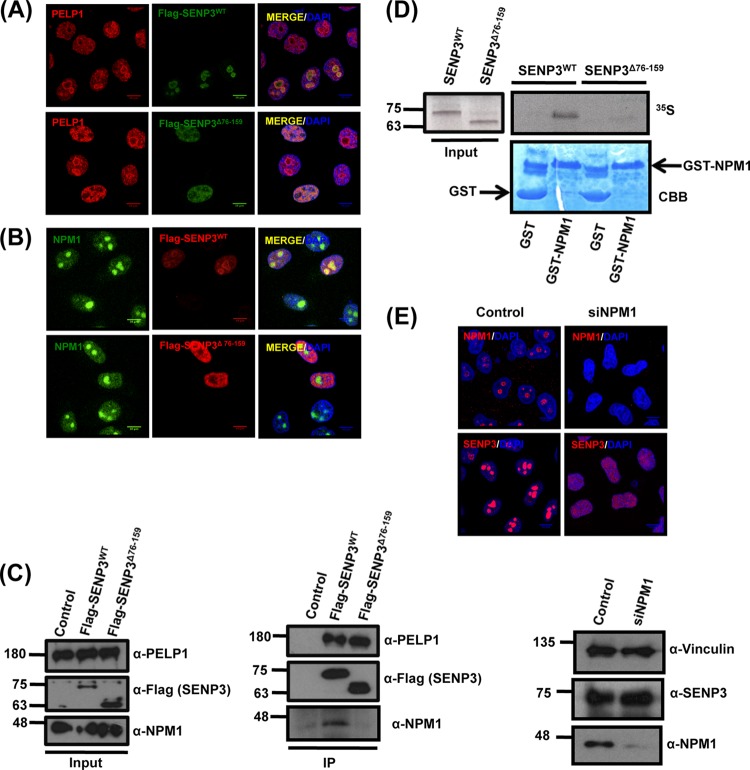
FIG 2.
NPM1 interaction is required for the nucleolar targeting of SENP3. (A) HeLa cells transiently expressing Flag-SENP3 or Flag-SENP3Δ76-159 were stained with anti-Flag and anti-PELP1 antibodies to determine their localization. (B) As described for panel A, except the cells were stained with anti-Flag and anti-NPM1 antibodies after transfection. (C) HeLa cells were transiently transfected with Flag-SENP3 or Flag-SENP3Δ76-159, and their interaction with NPM1 and PELP1 was determined after immunoprecipitation on Flag beads by immunoblotting using anti-Flag, anti-NPM1, and anti-PELP1 antibodies. (D) [35S]methionine-labeled SENP3 and SENP3Δ76-159 were generated by in vitro transcription/translation and used for GST pulldown assays using GST or GST-NPM1 bound to glutathione-Sepharose beads. After separation by SDS-PAGE, interactions were detected via autoradiography. (Upper) Autoradiography showing 35S-labeled SENP3 variants and their interaction with GST-NPM1. (Lower) Coomassie staining of the SDS-PAGE gel showing GST and GST-NPM1. (E) HeLa cells were either mock transfected or transfected with siRNA directed against NPM1. Seventy-two hours posttransfection, the cells were fixed and stained with anti-SENP3 or anti-NPM1 antibody in order to visualize their respective localizations through indirect immunofluorescence (top). Western blotting was performed with anti-SENP3 and anti-NPM1 antibodies to check for their proteins levels, and antivinculin was used as a loading control (bottom).