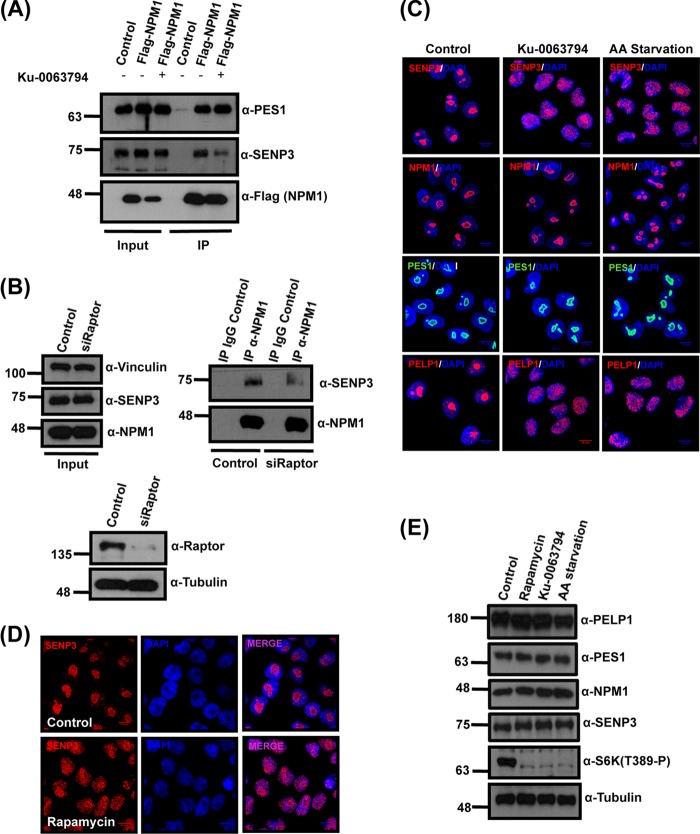
FIG 5.
mTOR affects SENP3 localization and SENP3-NPM1 interaction. (A) U2OS cells expressing Flag-NPM1 were either mock treated or treated with the mTOR inhibitor Ku-0063794. Subsequently, Flag-NPM1 was immunoprecipitated from these cells using Flag-agarose beads and separated by SDS-PAGE. Anti-Flag antibody was used to verify the immunoprecipitation of Flag-NPM1, and anti-SENP3 or anti-PES1 antibody was used to check for coimmunoprecipitation. (B) HeLa cells were transfected with either control siRNA or siRNA directed against Raptor, and 72 h later the cells were harvested for immunoprecipitation of the endogenous NPM1. (Upper right) The samples then were probed by immunoblotting with anti-NPM1 or anti-SENP3 antibody to check for NPM1 levels and coimmunoprecipitation of SENP3. (Upper left and lower) The proteins levels in control and knockdown samples were monitored by Western blotting with anti-NPM1, anti-SENP3, and anti-Raptor antibodies with antitubulin and antivinculin antibodies as loading controls. (C) HeLa cells were either mock treated, treated with Ku-0063794, or starved in Earle's balanced salt solution (EBSS) for 6 h and stained with anti-SENP3, anti-NPM1, anti-PES1, and anti-PELP1 antibodies to visualize their respective localization by indirect immunofluorescence. (D) SENP3 localization in HeLa cells was detected by immunofluorescence using anti-SENP3 antibody after treatment with rapamycin. (E) Immunoblotting of the HeLa cell lysates was done to check for changes in protein levels using anti-PELP1, anti-SENP3, anti-PES1, anti-S6K(T389-P), and anti-NPM1 antibodies after EBSS starvation or rapamycin or Ku-0063794 treatment. Antitubulin antibody was used as a loading control.