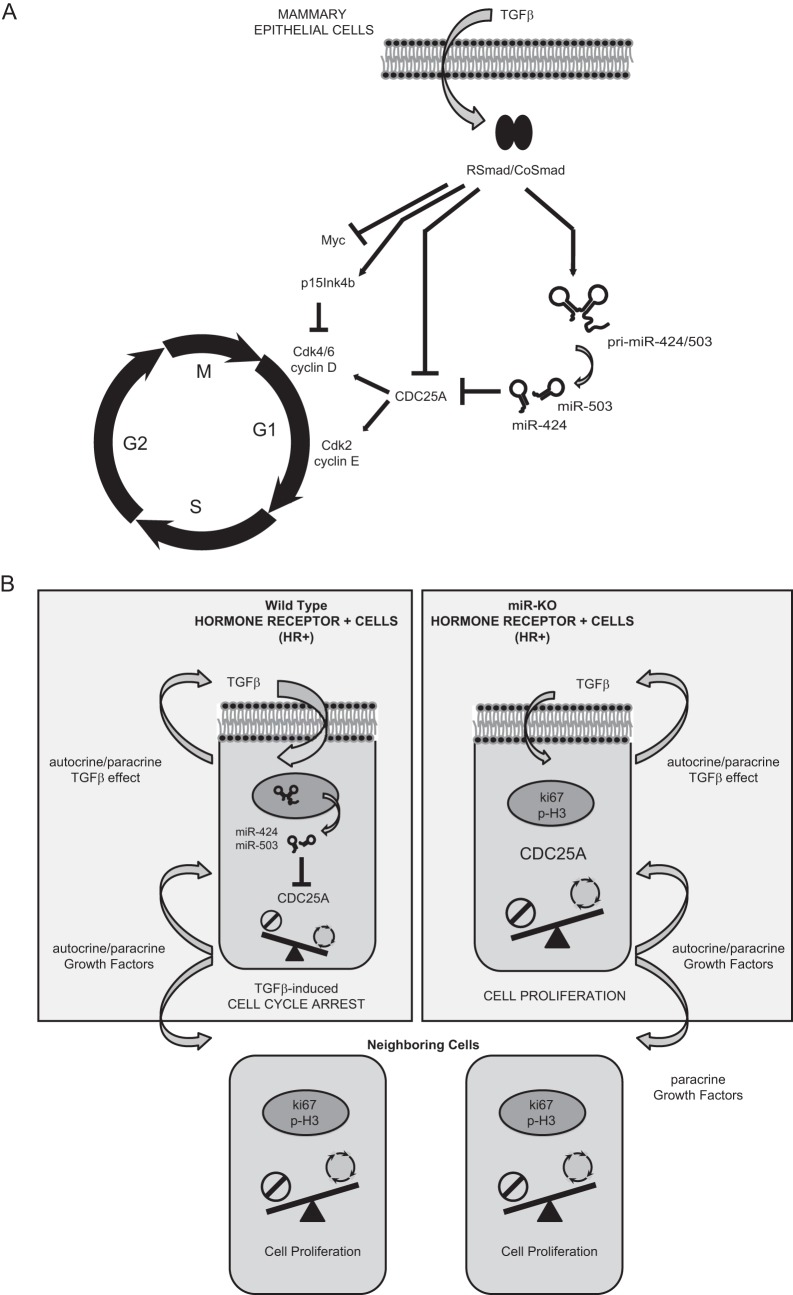
FIG 9.
Mechanistic model for miR-424/503 during TGF-β-mediated cytostasis in mammary epithelial cells. (A) General overview of the proposed molecular mechanistic model. When stimulated with TGF-β, mammary epithelial cells activate a signal transduction cascade triggered by SMAD proteins that aim to inhibit cell cycle progression by arresting cells in G1 phase. Induced expression of antiproliferative p15INK4B and the repression of c-Myc and CDC25A are well-known key factors associated with cell cycle arrest in response to TGF-β. We describe the upregulation of miR-424(322) and miR-503 by TGF-β as a novel mechanism that cooperates with transcriptional repression and proteasome-mediated degradation to reduce the expression level of CDC25A. (B) Proposed miR-424/503 functional activity at a cellular level. Under normal conditions, luminal/HR+ cells can foster proliferation of surrounding epithelial cells (ER−) through the secretion of locally acting growth factors. However, luminal/HR+ cells are themselves restrained from proliferation through an autocrine TGF-β cytostatic program that involves downregulation of CDC25A in part due to the increased expression of miR-424 and -503. In their absence, the inhibitory function of TGF-β is reduced, and the resulting increased CDC25A protein levels promote hyperproliferation of luminal/HR+ cells.