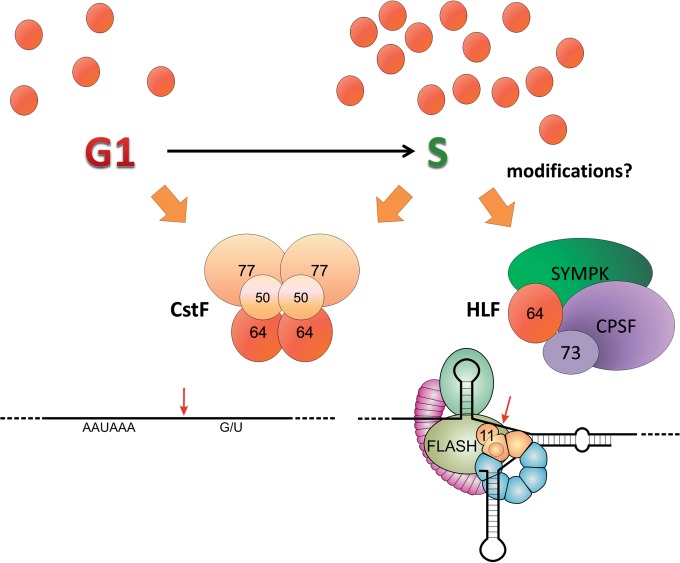
FIG 8.
Schematic illustration of CstF64's dual roles in cleavage and polyadenylation and histone RNA processing. CstF64 (red brown) can act in cleavage and polyadenylation (CPA) or histone RNA 3′ end processing as a component of, respectively, the hexameric CstF complex or the heat-labile factor (HLF) that additionally contains symplekin (SYMPK) and CPSF (of which the endonuclease CPSF73 is shown as a separate unit). The formation of these alternative complexes depends at least partly on mutually exclusive interactions of the CstF64 hinge domain with CstF77 or SYMPK (20, 33). Low CstF64 levels in the G1 phase may favor CstF formation and hence CPA. During the G1/S transition, increasing CstF64 levels and possibly posttranslational modifications may allow increased HLF formation. Tethering of HLF to the factors bound to a histone pre-mRNA processing site are mediated by symplekin and CstF64, which can both interact with a binding platform provided by FLASH (olive green) and the N terminus of the U7 snRNP component Lsm11 (sand brown). Binding of CstF64 to FLASH depends on the MEARA/G domain, as shown in Fig. 4, and possibly also on posttranslational modifications. Other histone pre-mRNA bound factors shown are stem-loop- or hairpin-binding protein (sea green) and 100-kDa zinc finger protein (violet). Further components of the CPA complex have been omitted for simplicity.