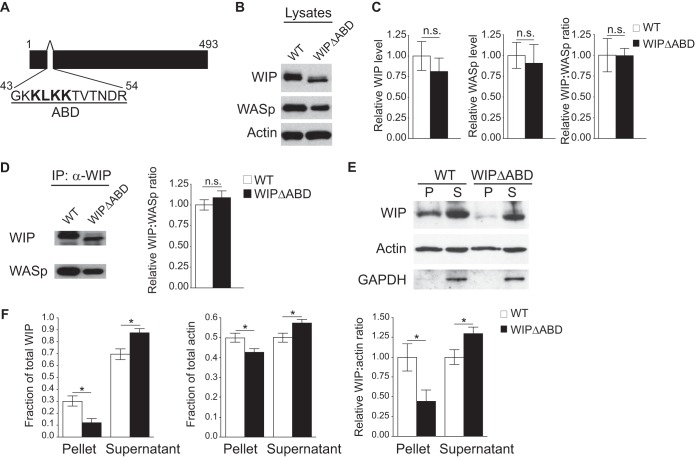
FIG 1.
The WIPΔABD mutant exhibits impaired cosedimentation with F-actin but a normal association with WASp. (A) Schematic representation of the WIP sequence with the deleted actin-binding domain (ABD) shown. (B) Representative Western blot of WIP, WASp, and actin in splenocyte lysates from WIPΔABD mice and WT controls. Similar results were obtained in five independent experiments. (C) Quantification of WIP and WASp levels relative to actin and of the WIP/WASp ratio in splenocytes from WIPΔABD mice and WT controls, normalized to a value of 1.0 for the WT controls (n = 5 for each group). (D) Representative Western blot analysis of WIP and WASp (left panel), and quantitative analysis of the WIP/WASp ratio (right panel) in WIP immunoprecipitates from splenocyte lysates of WIPΔABD mice and WT controls. The WIP/WASp ratio in WT controls was set at 1.0 (n = 3 for each group). (E) Representative Western blot analysis of WIP and actin in the high-speed centrifugation pellet (P) and supernatant (S) of splenocyte lysates from WIPΔABD mice and WT controls. GAPDH was used to rule out contamination of the P fraction with soluble proteins. Similar results were obtained in five independent experiments. (F) Quantitation of the fractions of WIP and actin, and of the WIP/actin ratio in the high-speed centrifugation pellet and supernatant of splenocyte lysates from WIPΔABD mice and WT controls. The WIP/WASp ratio in WT controls was set at 1.0 (n = 5 for each group). Columns and bars represent means ± the standard errors of the mean (SEM). *, P < 0.05; n.s., not significant.