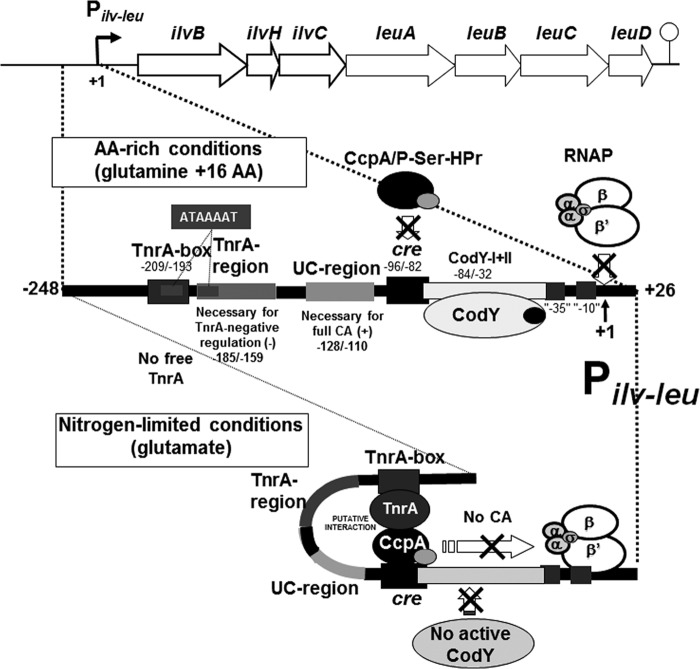
FIG 8.
The molecular mechanisms underlying CodY- and TnrA-negative regulation to negate CA exerted by the complex of CcpA and P-Ser-HPr. The ilv-leu operon is catabolite activated in the presence of rapidly metabolizable carbon sources such as glucose through binding of the complex of CcpA and P-Ser-HPr to the cre sequence (nt −96/−82); P-Ser-HPr is formed by HPr kinase, which is activated by fructose-bisphosphate, whose concentration increases during growth on rapidly metabolizable carbon sources such as glucose (7). The UC region (nt −128/−110) is necessary for full CA, but its function in CA is unknown. The binding of the complex to cre is displaced by tight binding of CodY to the CodY-I+II (nt −84/−32) site; isoleucine and GTP are bound to CodY for its activation on cell growth under AA-rich conditions. On the other hand, CA might be negated by the putative interaction of TnrA bound to the TnrA box (nt −209/−193) with the complex of CcpA and P-Ser-HPr to cre on DNA bending; TnrA becomes free in cells growing under nitrogen-limited conditions (11). Also, TnrA-negative regulation requires the TnrA region downstream of the TnrA box (nt −185/159), which likely has an unknown function in DNA bending for the interaction of TnrA and the complex. In addition, a direct repeat of ATAAAAT is shown in the TnrA box and region and is likely required for the TnrA-mediated negative regulation.