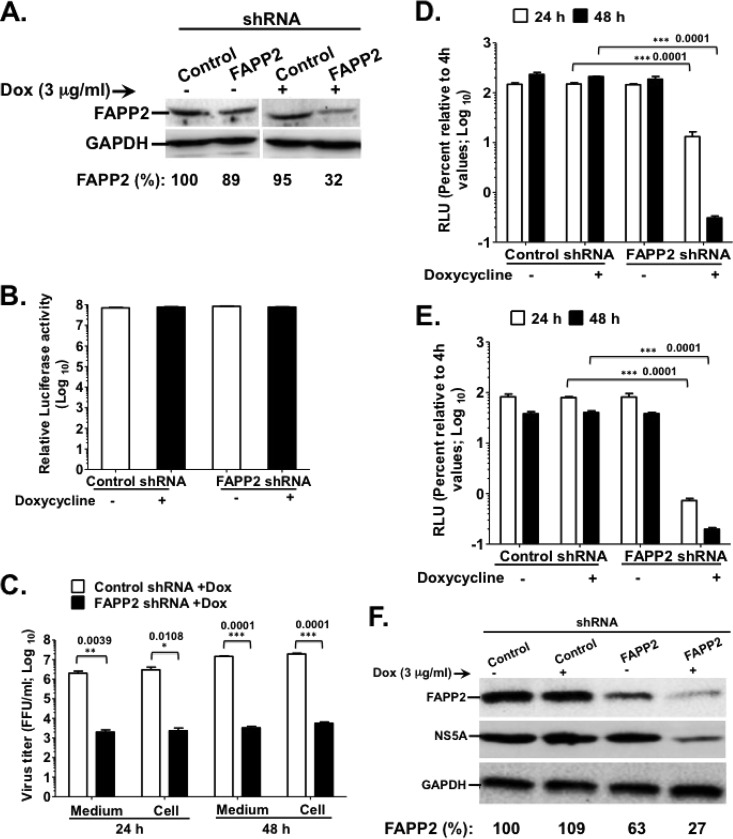
FIG 2.
FAPP2 function is required for HCV genome replication. (A) Control and FAPP2 shRNA-expressing cells were treated with 3 μg/ml doxycycline or left untreated. Forty-eight h posttreatment, cell lysates were separated by SDS-PAGE, followed by immunoblotting with αFAPP2 (1:1,000) and αGAPDH (1:8,000) antibodies. (B) Control and FAPP2 shRNA cells were treated as described for panel A. At 48 h posttreatment, cell viability was determined using the CellTiter-Glo luminescent cell viability assay. (C) Control and FAPP2 shRNA cells were induced with 3 μg/ml doxycycline. At 48 h postinduction, the cells were electroporated with 10 μg of HCV Jc1 RNA. At 24 h and 48 h posttransfection, cell-associated (Cell) and extracellular (Medium) viruses were collected. Virus titers were measured using a limiting-dilution assay (16, 20), and the results are expressed as focus-forming units (FFU)/ml. (D and E) Control and FAPP2 shRNA cells were grown for 48 h with or without doxycycline, followed by transfection with 10 μg of Luc-JFH1 (D) or Luc-Con1 (E) replicon RNA. At 4 h, 24 h, and 48 h posttransfection, cell lysates were collected and HCV replication efficiency was measured by luciferase reporter activity as reported previously (16, 20). RLU, relative light units. The values represent percent luciferase activity relative to 4-h values. (F) The cell extracts also were collected at 48 h posttransfection (D) for immunoblotting with FAPP2 (1:1,000)-, NS5A (1:8,000)-, or GAPDH (1:8,000)-specific antibody. The data are representative of at least two independent experiments with triplicate samples for panels B to E. *, P < 0.05 (statistically significant); **, P < 0.01 (very significant); ***, P < 0.001 (extremely significant).