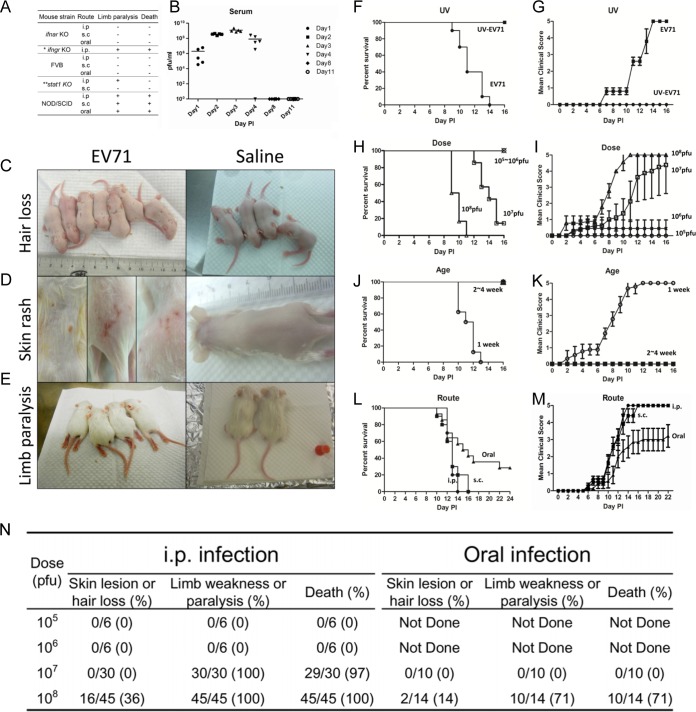
FIG 1.
Studies on various parameters that affect disease manifestations in immunodeficient mice infected with a clinical isolate of EV71 (strain F23, genotype B5). (A) Comparisons among various immune-deficient mouse models infected with EV71 via different routes (*, limb paralysis rate of ∼78%; **, limb paralysis rate of ∼31.3%). (B) One-week-old NOD/SCID mice were infected with 108 PFU of EV71 strain F23. Serum samples were collected from a group of five mice on days 1, 2, 3, 4, 8, and 11, respectively. Virus titers in serum were measured by plaque assays. (C through E) One-week-old NOD/SCID mice were infected with EV71 strain F23 (left) or normal saline (right). Disease manifestations, including hair loss (C), skin rash (D), limb paralysis (E), and death, were monitored daily. (F through M) Comparisons were made for different parameters of infection, including UV treatment (or no treatment) of EV71 (F and G), viral dose (≤106 versus 107 to 108 PFU/mouse) (H and I), host age (1 week versus 2 to 4 weeks) (J and K), and inoculation route (i.p., s.c., or oral) (L and M). Clinical scores were defined as follows: 0, healthy; 1, hair loss, wasting, or ruffled hair; 2, limb weakness; 3, paralysis in only 1 limb; 4, paralysis in 2 to 4 limbs; 5, death. Error bars indicate standard deviations for 6 mice in each group. (N) Summary of disease manifestations in NOD/SCID mice infected with EV71 at different viral doses and through different routes.