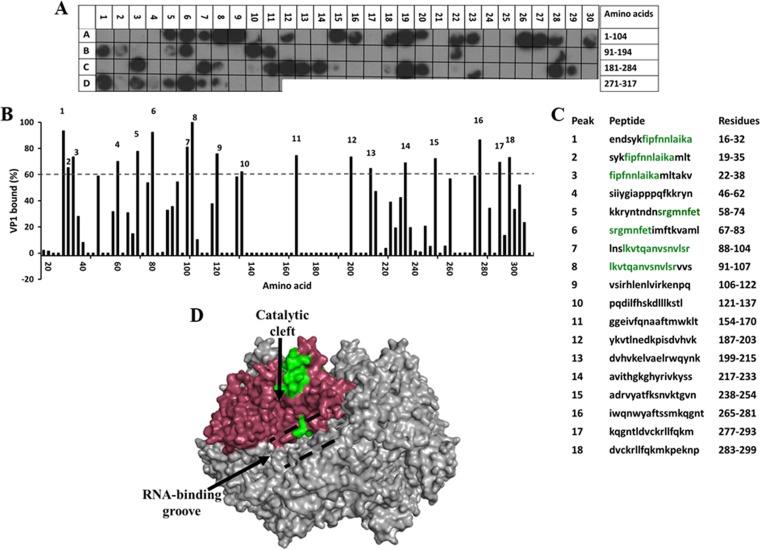
FIG 4.
Binding of the full-length SA11 VP1 to the peptide array of the SA11 NSP2 sequence. (A) Autoradiograph of the NSP2 peptide array probed by full-length VP1. The peptide array consists of spots of 17-residue peptides in the NSP2 sequence, starting from the N terminus (spot A1) and ending with the C-terminal peptide (D11), with the N-terminal residue of the peptide in each spot shifted by 3 residues from the previous spot along the NSP2 sequence. (B) Graph showing the relative intensity (y axis) of each spot (black bars) in the array with its position relative to the protein sequence (x axis). (C) The peptide sequences corresponding to the spots denoted 1 to 18 in panel B that showed intensities higher than the 60% cutoff. NSP2 residues present in at least two peptides that showed a ≥80% binding intensity (green) were mapped onto the known structure of NSP2 (pdb 1L9V). (D) Surface model of the NSP2 octamer (gray) with VP1-binding residues shown in green on monomer A (pink). The RNA-binding groove is shown by dashed black lines, and the catalytic cleft is indicated by a black arrow.