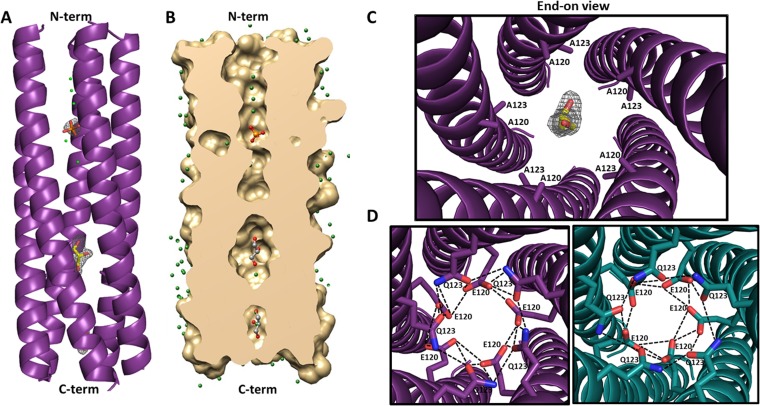
FIG 5.
Structure of the E120A/Q123A SA11 NSP4 CCD pentamer. (A) A side view of the ribbon representation of E120A/Q123A SA11 NSP4 CCD (residues 95 to 146) pentamer formed by five parallel helices (purple). The helical nature of all five chains extends from residues 95 (labeled as N-term on the top) to 137 (labeled as C-term on the bottom), with the last nine residues not clearly resolved in the structure. A phosphate molecule (orange sticks with oxygen shown in red) and two glycerol molecules (yellow sticks with oxygen shown in red) are depicted inside the channel. Fo-Fc difference map densities (3σ level) for the phosphate and glycerol molecules are shown in gray. Water molecules are shown as green spheres. (B) A longitudinal slice of the E/Q-CCD pentamer surface model (tan) revealing a cross section of the axial pore. A phosphate molecule is rendered as orange sticks, and two glycerol molecules are rendered as gray sticks, with oxygen shown in red. Water molecules are shown as green spheres. (C) Closeup of the end-on view of the mutated Ca2+-binding site, with A120 and A123 residues shown as purple sticks, a glycerol molecule shown as yellow sticks, and oxygen shown in red. Difference map density for the glycerol molecule is shown in gray. (D) Modeling of the E120 and Q123 residues into the E/Q-CCD pentamer structure and comparison with the ST3 CCD pentamer (PDB 3MIW) reported by Chacko et al. (14). Right, alanine residues at positions 120 and 123 in the E/Q-CCD were mutated back to E and Q (purple sticks, with nitrogen shown in blue and oxygen shown in red), respectively, as in the native SA11 sequence. The diameter of the pentamer accommodates all 10 residues and allows for formation of hydrogen bond interactions (dotted black lines) between the residues that are very similar to the interactions formed in the ST3 pentamer. Left, closeup end-on view of the hydrogen bond interaction (dashed black lines) between E120 and Q123 residues in the ST3 CCD pentamer (dark teal). E120 and Q123 residues are rendered as teal sticks, with nitrogen and oxygen atoms shown in blue and red, respectively.