FIG 5.
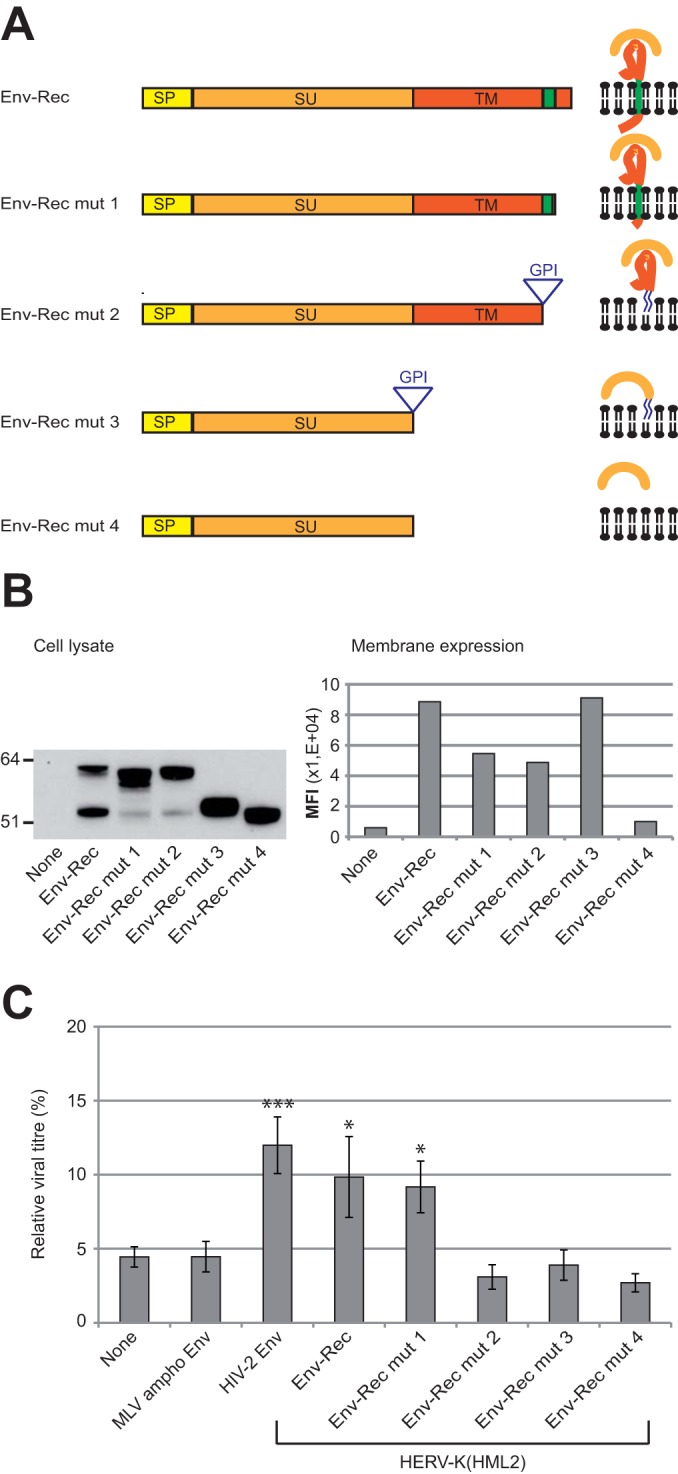
Characterization of HERV-K(HML2) Env domains necessary for inhibiting Tetherin activity. (A) Scheme of the different derivatives of HERV-K(HML2) Env-Rec. All modifications were designed to modify Env without altering the Rec ORF. The different domains of each construct are indicated: SP (signal peptide, yellow), SU (orange), and TM (red). HERV-K(HML2) Env-Rec mut1 corresponds to a C-terminal truncated version of the complete Env (41 aa deleted). HERV-K(HML2) Env-Rec mut2 has its membrane spanning (in green) and cytoplasmic domains replaced by a GPI anchor (the signal for the addition of the GPI anchor is depicted by a triangle in the scheme). HERV-K(HML2) Env-Rec mut3 consists of the SU subunit attached to the cell membrane by a GPI anchor. HERV-K(HML2) Env-Rec mut4 corresponds to the soluble surface subunit. For each construct, the expected protein structure is depicted on the right. (B) Expression levels of Env-Rec and derivatives were measured in transiently transfected 293T cells either by Western blotting on whole-cell lysates (left) or by FACS following cell surface staining (right). The histogram displays the median fluorescence intensities (MFI) of the whole population for each protein tested. (C) The efficiency of the different HERV-K(HML2) Env-Rec derivatives to antagonize Tetherin was assayed as described in the legend to Fig. 2A. The representation of results is the same as that described in the legend to Fig. 3A.
