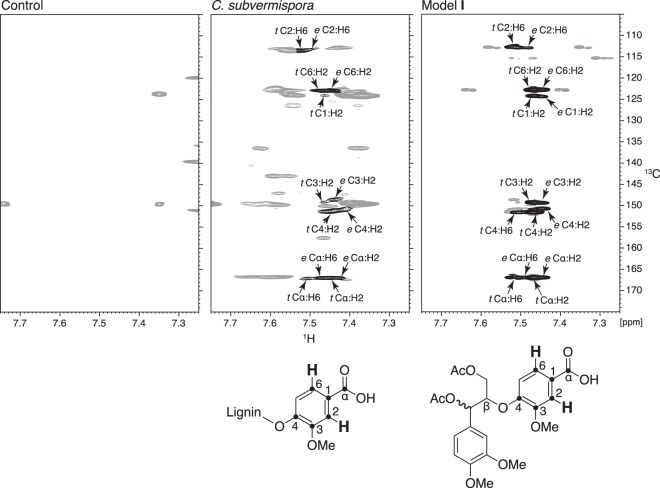
FIG 3.
1H-13C HMBC NMR spectra obtained on acetylated lignins from control and degraded spruce wood and from a threo/erythro mixture of lignin model I, showing correlations between aromatic H2/H6 and carbons two to three bonds away. Assigned cross-peaks are shown in black and are labeled to associate them with particular carbons and protons in lignin benzoic acids and in model I, which are depicted below their respective spectra. Each assignment states whether the contour is attributed to the threo (t)- or erythro (e)-diastereomer, followed by the numbered positions of the carbons and protons responsible. Unassigned contours are shown in gray. OMe, methoxyl.