FIG 1.
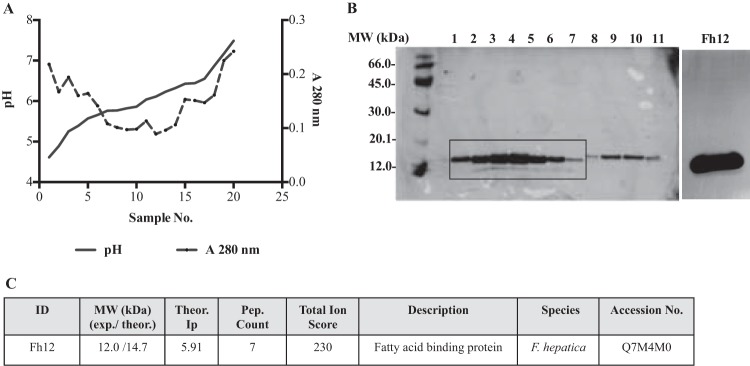
Purification of the native 12-kDa Fasciola hepatica fatty acid binding protein (Fh12). After molecular sieving chromatography using a Sephadex G-50 column, peak 2, which contains proteins of 12.0 to 20.0 kDa, was dialyzed against 1% glycine and subjected to successive fractionations by preparative isoelectric focusing (IEF) using ampholyte mixtures of pH 3 to 10 and pH 5 to 7 at a ratio of 1:4. Individual IEF fractions were harvested, and their pH and absorbance at 280 nm were measured. (A) Typical curve of pH versus A280 obtained in each IEF separation. (B) IEF fractions were analyzed by 12% SDS-PAGE, and the proteins were visualized by silver staining. Fractions 1 to 7, with a pH range of 4.2 to 5.9, were excised from the gel, analyzed by MALDI-MS/MS, pooled, and named Fh12. (C) The presence of fatty acid binding protein was identified by MALDI-MS/MS in the pooled fractions.
