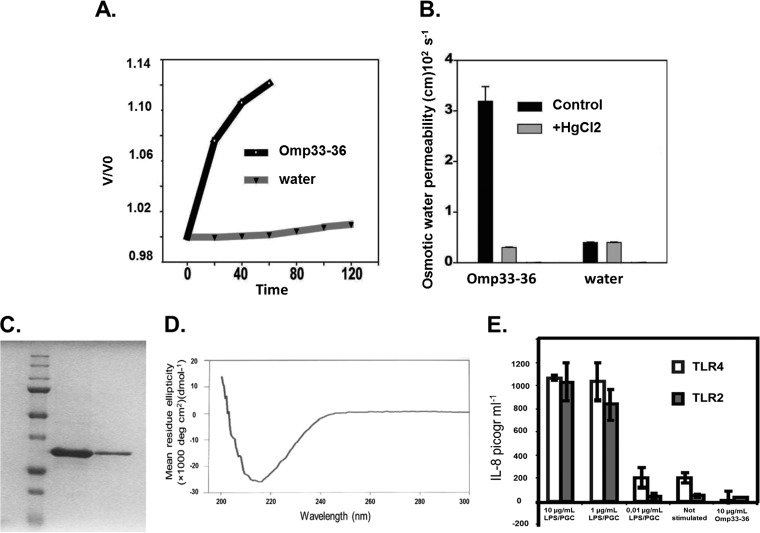
FIG 1.
(A) Omp33-36 acts as a channel (porin) for the passage of water. The x axis shows the time course of osmotic swelling of individual Xenopus oocytes injected with water or the cRNA of Omp33-36 from A. baumannii. Oocytes in ND96 were perfused at time zero with a 5-fold dilution of ND96 in distilled water. On the y axis, V0 is the initial cell volume and V is the cell volume after treatment. (B) The porin is an aquaporin. The osmotic permeability (Pf) to water of individual oocytes injected with water or the cRNA of Omp33-36 from A. baumannii (bars labeled Omp33-36) was determined. Oocytes in ND96 were perfused at time zero with a 5-fold dilution of ND96 in distilled water (bars labeled water). The Pf values reflect the volume changes determined in three different oocytes. When indicated, the inhibition assay was performed in the presence of mercury (50 μM HgCl2, 10 min preincubation) dissolved in the same distilled water used for diluting ND96. (C) Purification of Omp33-36. A Coomassie blue-stained gel of purified Omp33-36 from A. baumannii is shown. SDS-PAGE on a 12% acrylamide gel did not reveal any bacterial contaminants in 16 μg of purified Omp33-36. (D) β-Barrels as a secondary structural feature of the porin. The circular dichroism spectrum of Omp33-36 in Tris-HCl, pH 8, 150 mM NaCl, and 0.06% LDAO is shown. The Omp33-36-LDAO spectrum revealed β-barrels as the secondary structure. (E) Absence of contaminants in Omp33-36. The results of the IL-8 ELISA performed with the culture medium of HEK293/TLR4 cells stimulated for 4 h with 10, 1, or 0.01 μg of E. coli LPS/ml, not stimulated, or stimulated with 10 μg pure Omp33-36/ml are shown. HEK293/TLR2 cells were challenged in the same way, except that commercial peptidoglycan was used. Pure Omp33-36 did not contain any bacterial contaminants that triggered TLR2 or TLR4 activation.