Abstract
The rapid increase in Mycobacterium tuberculosis resistance to ethambutol (EMB) threatens the diagnosis and treatment of tuberculosis (TB). We investigated the role of mutations in the embC-embA intergenic region (IGR) in EMB-resistant clinical strains from east China. A total of 767 M. tuberculosis clinical strains were collected and analyzed for their drug susceptibility to EMB using the MGIT 960 system and MIC assay, and the embC-embA IGRs of these strains were sequenced. The transcriptional activity of the embC-embA IGR mutations was examined by reporter gene assays in recombinant Mycobacterium smegmatis strains, and the effect of IGR mutations on its binding to EmbR, a transcription regulator of embAB, was analyzed by gel mobility shift assays. Correlation coefficient analysis showed that the embC-embA IGR mutation is associated with EMB resistance. The clinical strains carrying IGR mutations had a much higher level of embA and embB mRNA as well as higher MICs to EMB. IGR mutations had higher transcriptional activity when transformed into M. smegmatis strains. Mutated IGRs bound to EmbR with much higher affinity than wild-type fragments. The sensitivity of molecular drug susceptibility testing (DST) with IGR mutations as an additional marker increased from 65.5% to 73.5%. Mutations of the embC-embA IGR enhance the binding of EmbR to the promoter region of embAB and increase the expression of embAB, thus contributing to EMB resistance. Therefore, identification of IGR mutations as markers of EMB resistance could increase the sensitivity of molecular DST.
INTRODUCTION
Tuberculosis (TB) remains a major global health problem. It affects millions of people each year and is the second leading cause of death from an infectious disease worldwide. In 2012, an estimated 8.6 million people developed TB, and 1.3 million died from the disease. The emergence of drug-resistant strains of Mycobacterium tuberculosis, especially those that are multidrug resistant (MDR) and extensively drug resistant (XDR), has posed a serious threat to global TB control programs (1). An estimated 3.6% of new patients and 20.2% of previously treated patients have MDR-TB, and there were 450,000 new cases of MDR-TB worldwide in 2012 (2). Given the alarming rise of drug-resistant TB, the identification of drug resistance genes is critical for the detection and treatment of TB. Much progress has been made to identify gene mutations in specific loci of the M. tuberculosis genome as the molecular basis for TB drug resistance and as drug targets for the development of anti-TB drugs. Although an association of mutations in these resistance genes with drug resistance has been observed, the exact role these genes play in the development of drug resistance is not fully understood. Furthermore, a significant number of anti-TB drug-resistant strains do not carry these mutations, suggesting that unknown gene mutations or variations are involved in the development of anti-TB drug resistance.
Ethambutol (EMB) is an essential first-line anti-TB drug that inhibits the biosynthesis of cell wall arabinogalactan (3). The resistance rate to EMB has gradually increased in some regions and approaches 50% in re-treated TB patients (4–6). In China, the resistance rate to EMB increased from 6.52% in 2007 to 17.18% in 2010 (7). Resistance to EMB is caused by mutation of the embCAB operon (embC, embA, and embB) that encodes membrane-associated arabinosyltransferases involved in the synthesis of cell wall arabinogalactan. Approximately 50% to 70% of EMB-resistant clinical M. tuberculosis isolates carry a mutation in a relatively short region in embB, primarily at codons 306 (embB306), 406 (embB406), and 497 (embB497), which therefore represent promising diagnostic markers for the rapid detection of EMB resistance (8–14). However, allelic-exchange studies indicate that embB306, embB406, and embB497 mutations only modestly increase resistance to EMB in M. tuberculosis (15, 16). Some mutations of the embC-embA intergenic region (IGR) have been identified in EMB-resistant clinical strains, but the molecular basis of these mutations in the regulation of EMB resistance is not well characterized (10, 17). In this study, we analyzed the mutations in embC-embA IGR in 767 clinical isolates of M. tuberculosis strains and verified the effects of mutations in embC-embA IGR on EMB resistance in M. tuberculosis.
MATERIALS AND METHODS
Strains.
A total of 767 M. tuberculosis clinical strains were randomly collected from patients with pulmonary tuberculosis. All of the pulmonary tuberculosis patients were from east China. The clinical strains were identified as M. tuberculosis using PCR for the IS6110 sequence (18).
DST.
All isolates were initially classified as EMB resistant or susceptible in routine diagnostic laboratories by the Bactec MGIT 960 method (5 μg/ml) (19). All strains were cultured in a mycobacterial growth indicator tube (MGIT) with the Bactec MGIT 960 growth supplement (Becton Dickinson Diagnostic Systems, MD). We used the MGIT 960 instrument and the EpiCenter software package (Becton Dickinson Diagnostic Systems, MD). The standard protocol for susceptibility testing in MGIT 960 was strictly followed, as recommended for primary drugs. The Bactec MGIT 960 drug susceptibility testing (DST) supplement (0.8 ml) (oleic acid-albumin-dextrose-catalase), 100 μl of the drug stock solution, and 0.5 ml of the suspension containing M. tuberculosis were added to an MGIT tube. The growth control did not contain the drug stock solution. DST sets were entered into the Bactec MGIT 960 instrument and continuously monitored until a susceptible or resistant result was obtained. The DST set results were reported by the instrument (determined by the software algorithms, after the growth control became positive).
MIC testing.
To determine EMB MICs, susceptibility testing was performed with the microplate alamarBlue assay (MABA) using Middlebrook 7H9 medium (Becton Dickinson Diagnostic Systems, Sparks, MD), including a 10% albumin-dextrose-catalase (ADC) supplement and EMB at concentrations of 0, 0.25, 0.5, 1, 2, 4, 8, 16, and 32 μg/ml, with reference to the method of Franzblau et al. (20).
PCR and sequencing.
One milliliter of M. tuberculosis suspension collected from an MGIT 960 control tube was transferred to a 1.5-ml tube and centrifuged at 10,000 × g for 5 min. The supernatant was discarded, and the sediment was resuspended in 50 μl DNA extraction solution (0.04% NaOH, 0.1% SDS, 15% Chelex-100 chelating resin) and mixed by vortexing. Subsequently, the tube was incubated at 100°C for 15 min and centrifuged at 13,000 × g for 10 min after it had cooled. Finally, the supernatant was transferred to a fresh1.5-ml tube and preserved at −20°C until used as a PCR template.
The embC-embA IGR was amplified with primer 1 (5′-GGTTGACGCCTTACTACCC-3′) and primer 2 (5′-CCACGACGACCGTGTCC-3′). The embB mutation hot region (including codons 306, 406, and 497) was amplified with primer 3 (5′-CTGAAACTGCTGGCGATCAT-3′) and primer 4 (5′-ATAGCGCGGTGATCAAAAAG-3′). These primers were designed by Primer-BLAST software with reference to embABC (GenBank accession no. NC_000962.2) gene sequences of M. tuberculosis H37Rv. The sizes of the amplified fragments were 535 bp for embC-embA IGR and 997 bp for embB. The PCR products were purified and sequenced at the Beijing Genomics Institute (BGI [Shenzhen, China]). The DNA sequences were analyzed with MegAlign 5.01 software (demonstration system; DNAStar, Inc., Madison, WI).
Quantitative real-time PCR of embAB mRNA.
All of the strains with embC-embA IGR mutations and 15 randomly selected EMB-susceptible strains without embC-embA IGR and embB mutations were cultured in a mycobacterial growth indicator tube (MGIT) with Bactec MGIT 960 growth supplement. After 1 week, all strains were in log phase. The strains were collected, and total RNA was extracted immediately. RNA was extracted as described previously (21). The genomic DNA was removed using the PrimeScript reverse transcription (RT) reagent kit with gDNA Eraser (TaKaRa Biotechnology Co., Ltd. Inc., Dalian, China). RT was carried out using random primers. The reaction was carried out with SBGR reverse transcription reagents (Tiangen Biotech Co., Ltd., Beijing, China). The primers for embA (5′-CTACGGGGAGAACAACCTGG-3′ and 5′-CCACTGCAGTTTCAGGGACT-3′) and embB (5′-ATGCTGCGGATCTTGGTGCG-3′ and 5′-CCGATTTTGGCGCGAACCCT-3′) and the primers for 16S rRNA (5′-GTCAAGTCATCATGCCCCTT-3′ and 5′-CACCTTCGACAGCTCCCTCC-3′) were used in real-time PCR assays. embAB expression was normalized against the 16S rRNA housekeeping gene.
Analysis of promoter activity.
The embC-embA IGR of M. tuberculosis was amplified using primer 5 (5′-CGCTTCTAGAAGCGGTTGACGCCTTACTAC-3′) and primer 6 (5′-TCTGGATCCAGATCGCTCATTACCGTCGT-3′) with XbaI and HindIII restriction endonuclease sites and was cloned upstream of the promoterless lacZ gene of vector pMC210 (a kind gift from Xiaoyong Fan). The recombinant pMC210-IGR plasmid was isolated and the sequence verified. Plasmids pMC210-IGR with the wild-type and different mutant types of embC-embA IGR were electroporated into Mycobacterium smegmatis. Kanamycin-resistant transformants were isolated. Three independent transformants for each were selected for promoter activity determinations.
β-Galactosidase activity was assayed in vitro in mycobacteria (22). Briefly, the recombinant mycobacterial strains were grown in complete Middlebrook 7H9 broth to the log phase, and the optical density at 600 nm (OD600) was measured. One hundred microliters of cells was added to 900 μl of Z buffer (6 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, 50 mM β-mercaptoethanol) and permeabilized with 50 μl of 0.1% SDS and 100 μl of chloroform. The mixtures were vortexed and then incubated for 15 min at room temperature. Two hundred microliters of substrate o-nitrophenyl-β-d-galactosidase (ONPG [4 mg/ml in 100 mM KH2PO4, pH 7.0]) was added, and the time taken for yellow color development was noted. The reaction was halted by addition of 0.2 ml of 2.5 M Na2CO3. The solutions were centrifuged to remove cell debris, and the optical density at 420 nm was measured. The β-galactosidase activity in modified Miller units was calculated with the following formula: (OD420 × 1,000)/(t × v × OD600), in which t is the incubation time in minutes, v is the volume of culture in ml, and OD420 or OD600 is the optical density at 420 or 600 nm, respectively.
Gel mobility shift assay (EMSA).
The electrophoretic mobility shift assay (EMSA) was performed as follows. The expression and purification of EmbR and the phosphorylation of EmbR by PknH followed the method of Molle et al. (23). For the protein-DNA binding assay, a DNA fragment was purified as a PCR product. The primers were 5′-GAAGTGCCGTGGCCACCGAC-3′ and 5′-CGTGCGATCCGGTGAGATCG-3′. The PCR products representing different promoter regions were incubated with constant amounts of phosphorylated EmbR at 4°C for 30 min in buffer containing 10 mM Tris-HCl (pH 7.0), 1 mM dithiothreitol, 1 mM EDTA, and 10% glycerol in a total volume of 10 μl. After incubation, complexes and free DNA were resolved by 5% nondenaturing polyacrylamide gels with a running buffer containing 40 mM Tris-HCl (pH 7.8), 20 mM sodium acetate, and 1 mM EDTA. The gels were then stained with SYBR green, and the band intensity was analyzed by Gel-Pro analysis software (Media Cybernetics, Inc.).
Data analysis.
Data analysis was carried out using GraphPad Prism 5 (GraphPad Software, Inc., CA). The mRNA transcriptional level and the MIC of EMB were compared with the Mann-Whitney U test. Comparison of sensitivities, specificities, positive predictive values (PPV), and negative predictive values (NPV) was performed using a chi-square test.
RESULTS
embB mutations in EMB-resistant strains.
We collected 767 epidemiologically unrelated M. tuberculosis clinical strains from TB patients in east China. Of the 767 clinical strains, 275 strains were found to be EMB resistant. In the 275 EMB-resistant strains, only 180 (65.5%) carried known EMB resistance-associated embB mutations at codons 306, 406, and 497. Sixteen (5.8%) of the EMB-resistant strains carried other embB site nonsynonymous mutations (Table 1).
TABLE 1.
Mutation of embA and embB in M. tuberculosis clinical strains
|
embB mutation codon substitution |
No. of strains with EMB DST resulta: |
embA mutation nucleotide substitution or deletion |
No. of strains with EMB DST resulta: |
||||
|---|---|---|---|---|---|---|---|
| Site(s) | Pattern | S | R | Site | Pattern | S | R |
| 306 | M-I | 23 | 34 | −12 | C-T | 2 | |
| −16 | C-A | 1 | |||||
| −16 | C-T | 1 | |||||
| −16 | C-G | 7 | |||||
| M-L | 6 | 10 | −11 | C-A | 2 | ||
| −12 | C-T | 1 | |||||
| −16 | C-A | 2 | |||||
| −16 | C-T | 1 | |||||
| M-V | 13 | 90 | −11 | C-A | 1 | ||
| −16 | C-T | 2 | |||||
| −16 | C-G | 1 | |||||
| 406 | G-S | 7 | 2 | −11 | C-T | 1 | |
| G-R | 1 | ||||||
| G-D | 11 | 3 | −12 | C-T | 1 | ||
| G-A | 4 | 15 | −16 | C-T | 1 | ||
| −16 | C-G | 3 | |||||
| 497 | G-C | 1 | |||||
| Q-P | 1 | ||||||
| Q-R | 3 | 7 | −16 | C-T | 1 | 1 | |
| −41 | A del | 3 | |||||
| Q-K | 1 | 5 | |||||
| 306 and 406 | M-I and G-D | 3 | |||||
| M-I and G-A | 2 | ||||||
| M-V and G-D | 2 | ||||||
| 306 and 497 | M-I and Q-F | 1 | |||||
| M-I and Q-R | 1 | ||||||
| 296 and 306 | N-I and M-I | 1 | |||||
| 304 and 306 | L-V and M-I | 1 | |||||
| 360 and 406 | V-A and G-A | 1 | |||||
| 246 | G-R | 1 | 4 | ||||
| 288 | L-V | 1 | |||||
| 296 | N-S | 4 | |||||
| 319 | Q-R | 1 | −12 | C-T | 1 | ||
| 328 | D-G | 1 | |||||
| 328 | D-F | 1 | |||||
| 328 | D-Y | 1 | 1 | ||||
| 330 | F-L | 1 | 4 | ||||
| 334 | Y-H | 1 | 0 | ||||
| 354 | D-A | 3 | 3 | −12 | C-T | 1 | |
| 380 | S-N | 1 | |||||
| 397 | P-R | 1 | |||||
| 397 | P-S | 1 | −16 | C-T | 1 | 0 | |
| 400 | N-S | 1 | |||||
| 409 | A-P | 1 | |||||
| 445 | E-R | 1 | |||||
| 446 | L-S | 1 | |||||
| 466 | L-W | 1 | |||||
| Detection region | wtb | −8 | C-T | 1 | |||
| −11 | C-A | 3 | |||||
| −12 | C-T | 1 | 9 | ||||
| −16 | C-T | 6 | |||||
| −16 | C-G | 1 | |||||
| −27 | T del | 1 | |||||
R, resistance; S, susceptibility.
wt, embB in the detection region was wild type.
embC-embA IGR mutations in M. tuberculosis clinical strains.
Among the 767 clinical strains, 57 strains (7.43%) were found to carry IGR mutations, located at positions −8, −11, −12, −16, −27, and −41 upstream of embA (Fig. 1 and Table 2). Of the 57 clinical strains carrying IGR mutations, 52 (91.22%) were found to be EMB resistant, as determined by the Bactec MGIT 960 method (5 mg/ml) (19). In 275 EMB-resistant strains, 149 strains carried only the embB mutation (e.g., site 306, 406, or 497), 31 strains carried both embC-embA IGR and embB mutations, and 22 strains carried only embC-embA IGR mutations. Correlation coefficient analysis of all 767 clinical strains showed that the embC-embA IGR mutation is associated with EMB resistance (r = 0.3375, P < 0.0001), suggesting that mutations of IGR may play a role in EMB resistance.
FIG 1.
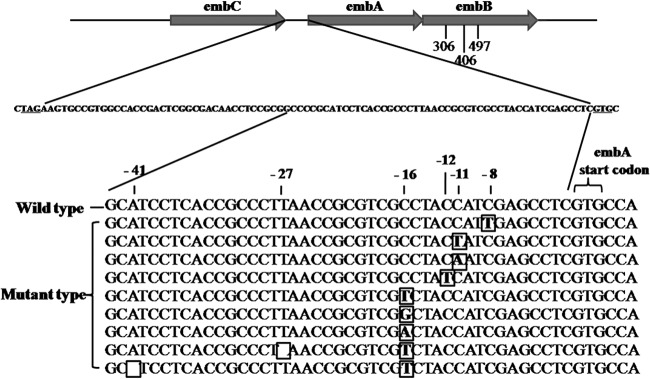
Alignment of the promoter sequence of embA in M. tuberculosis clinical isolates. −8, −11, −12, −16, −27, and −41 mutations of the embA promoter were identified in M. tuberculosis clinical isolates resistant to EMB. Mutations are marked in boldface in a box; a blank box indicates nucleotide deletion.
TABLE 2.
Mutation of the embC-embA IGR in M. tuberculosis clinical strains
| Mutation sites | Mutation pattern | No. of strains | MGIT EMB DST resulta | Median MIC, μg/ml (interquartile range [%]) |
|---|---|---|---|---|
| −8 | C-T | 1 | R | 4 (4–4) |
| −11 | C-A | 6 | R | 8 (2–8) |
| C-T | 1 | R | 4 (4–4) | |
| −12 | C-T | 14 | R | 4 (4–8) |
| C-T | 2 | S | 4 (4–4) | |
| −16 | C-G | 12 | R | 8 (2–16) |
| C-T | 12 | R | 4 (4–8) | |
| C-T | 2 | S | 4 (4–4) | |
| C-A | 3 | R | 8 (8–8) | |
| −27 | T del | 1 | R | 2 (2–2) |
| −41 | A del | 3 | R | 8 (8–8) |
R, resistance; S, susceptibility.
embAB mRNA transcription in clinical isolates.
To examine whether the IGR mutation is associated with the expression of embAB, the mRNA levels of embA and embB in all EMB-resistant clinical strains with embC-embA IGR mutations and 15 randomly selected EMB-susceptible strains without embC-embA IGR and embB mutations were analyzed by RT-PCR (Fig. 2). The transcription levels of embA and embB mRNA in EMB-resistant strains with only embC-embA IGR mutations were higher than those in EMB-susceptible stains without embC-embA IGR or embB mutations (P = 0.0011, P = 0.0003) by the Mann-Whitney U test. In EMB-resistant strains, the levels of embA and embB mRNA with only embC-embA IGR mutations were higher than those in EMB-resistant strains with only the embB mutation (P < 0.0001, P = 0.0006). The levels of embA and embB mRNA with both embC-embA IGR mutations and embB mutations were higher than those with only embB mutations (P = 0.0002, P = 0.0075). These results suggest that embC-embA IGR mutations may increase the transcription of downstream embA and embB.
FIG 2.
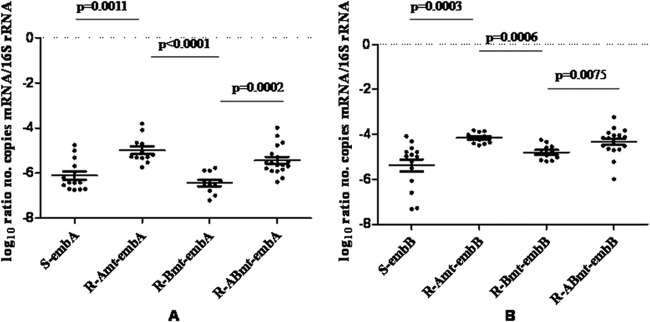
embC-embA IGR mutations lead to increased embA and embB mRNA expression in clinical strains. R, resistant to EMB; S, susceptible to EMB; Amt, only embC-embA IGR mutation; Bmt, only embB mutation; Abmt, both embC-embA IGR and embB mutations.
Effect of embC-embA IGR mutations on resistance to EMB.
We compared the MICs to EMB of those M. tuberculosis clinical strains with simultaneous embC-embA IGR and embB mutations by the microplate alamarBlue assay (20). The MICs of EMB of those strains with both embC-embA IGR mutations and embB mutations were much higher than those of strains with only an embB mutation (P = 0.0004) (Fig. 3). However, only the MICs of clinical strains with an embC-embA IGR mutation were similar to those of strains with only an embB mutation (P = 0.5313).
FIG 3.

embC-embA IGR mutations confer a higher resistance to EMB in clinical strains. pAmt, mutation in embC-embA IGR; Bmt, mutations in embB site 306, 406, or 497; pAwt, wild type in embC-embA IGR; Bwt, wild type in embB site 306, 406, or 497; R, EMB-resistant strains; S, EMB-susceptible strains.
The transcriptional activity of promoters of embAB in M. smegmatis.
To further analyze whether IGR mutations affect transcriptional activity, the IGR mutation region was cloned into the vector pMC210 upstream of the promoterless lacZ gene of vector pMC210 (19). The recombinant M. smegmatis strains transformed with embC-embA IGR mutation construct vectors (mutations of −8C to T [−8C-T], −11C-A, −11C-T, −12C-T, −16C-A, and −16C-T and the −27T deletion) had higher levels of β-galactosidase activity than the recombinant M. smegmatis with wild-type embC-embA IGR construct vectors (Fig. 4). Of all mutant types of embC-embA IGR, the −16C-A mutant had the highest level of β-galactosidase activity, which was almost 4-fold higher than that of the wild type.
FIG 4.
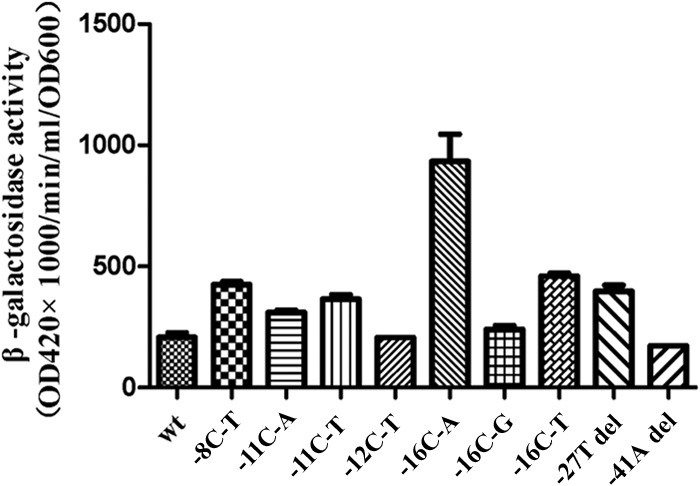
embC-embA IGR mutations as promoters of embA and embB increase the transcriptional activity. β-Galactosidase activities in M. smegmatis recombinants with mutant embC-embA IGR mutation construct vectors (−8C-T, −11C-A, −11C-T, −16C-A, −16C-T, and −27T deletion) were higher than those in the recombinant with the wild-type construct vector (P = 0.0049, 0.0049, 0.0024, 0.0049, 0.0049, and 0.0049, respectively).
Binding activity of EmbR to an embC-embA IGR fragment.
EmbR is a multidomain protein possessing three major domains: a DNA binding winged helix-turn-helix (W-HTH) domain, a bacterial transcription activation domain, and a forkhead-associated (FHA) domain (23). Phosphorylation of EmbR by mycobacterial serine/threonine kinase PknH enhances its DNA binding activity toward promoter regions of embCAB, thus regulating transcription of embCAB genes in M. tuberculosis (24). Interestingly, a mutation in the EmbR FHA domain was shown to be associated with EMB resistance (10). EMSA revealed that pEmbR binds to wild-type embC-embA IGR fragments with much lower affinity than other mutated embC-embA IGR fragments. This result suggested that mutant embC-embA IGR increased the binding activity of EmbR to embC-embA IGR (Fig. 5).
FIG 5.
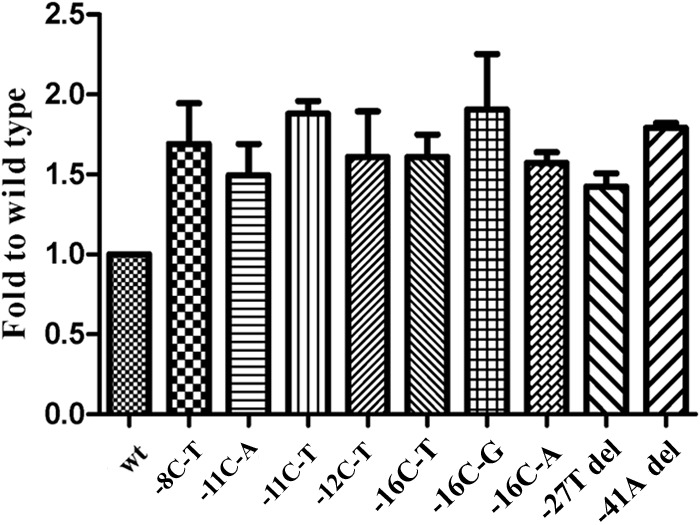
Binding activity of EmbR to embC-embA IGR. EmbR binds to wild-type embC-embA IGR fragments with much lower affinity than the mutant (−8C-T, −11C-A, −11C-T, −12C-T, −16C-A, −16C-T, −16C-G, −27T deletion and 41A deletion) embC-embA IGR fragments (P = 0.0107, 0.0172, 0.0020, 0.0150, 0.0059, 0.0013, 0.0342, 0.0090, and 0.0092, respectively).
The value of the embC-embA mutation in the diagnosis of molecular DST to EMB.
With reference to the MGIT 960 method, if embB mutations (including only known sites related to EMB resistance: codons 306, 406, and 497) were used as a judgment standard of molecular DST to EMB, the sensitivity, specificity, and accuracy in this study were 65.5% (180/275), 86.0% (423/492), and 78.6% (603/767). The positive predictive value (PPV) and the negative predictive value (NPV) were 72.3% (180/249) and 81.7% (423/518), respectively. If embC-embA IGR mutations were used as a reference standard of molecular drug susceptibility testing for EMB, the sensitivity, specificity, accuracy, PPV, and NPV were 19.3% (53/275), 99.2% (488/492), 70.5% (541/767), 93.0% (53/57), and 68.7% (488/710), respectively. If either of the embB or embC-embA IGR mutations was used as a reference standard of molecular DST for EMB, the sensitivity, specificity, accuracy, PPV, and NPV were 73.5% (202/275), 85.4% (420/492), 81.1% (622/767), 73.7% (202/274), and 85.2% (420/493), respectively. The PPV of the embC-embA IGR mutations was significantly higher than that of the embB mutations (χ2 = 9.895, P = 0.001).
DISCUSSION
With the rapid increase in EMB resistance in M. tuberculosis clinical strains, a rapid and accurate method is urgently needed for the diagnosis of EMB resistance. Molecular DST has shown good performance in the diagnosis of rifampin resistance, but the sensitivity and specificity of embB-based molecular DST of EMB resistance are lower, suggesting that other gene mutations are involved in EMB resistance. Previous studies by several groups have detected mutations in embC-embA IGR in strains from the United States, the former Soviet Union, Kuwait, Germany, and Uzbekistan but found no epidemiological correlation of these mutations with EMB resistance, possibly due to there being fewer clinical strains showing mutations in embC-embA IGR (10, 25, 26). In this study, we found embC-embA IGR mutations in clinical EMB-resistant strains from east China, suggesting a wide distribution of embC-embA IGR mutations across the world. Moreover, our analysis of a large number of clinical strains showed that mutations of embC-embA IGR were highly related to resistance to EMB in M. tuberculosis clinical strains.
Overexpression of embAB is associated with high-level EMB-resistant arabinosyltransferase activity (27). In our study, a higher level of embA and embB mRNA expression was found in strains with embC-embA IGR mutations than in strains without embC-embA IGR mutations, suggesting that the mutations in the embC-embA IGR may increase the transcriptional level of embA and embB. Indeed, the promoter activities of most of the mutant embC-embA IGRs were higher than those in the wild type, as assayed by a β-galactosidase activity reporter system in M. smegmatis. Of all 10 mutant genotypes of embC-embA IGR, the promoter activity of the −16C-A mutation is the highest. These results suggest that mutations of embC-embA IGR may increase the expression of embAB, thus contributing to EMB resistance.
It was reported that mutations in embB codons 306, 406, and 497 only cause EMB resistance at a low level of EMB (15, 16). Safi et al. reported that synonymous mutations in Rv3792 increased the expression of embC to increase the resistance level of EMB (28). We found that the MIC values for EMB in clinical strains with mutations in both embC-embA IGR and embB were significantly higher than those in strains carrying only the embB mutation. Moreover, the levels of embA and embB mRNA with both embC-embA IGR mutations and embB mutations were higher than those with only the embB mutation. Thus, mutations in embC-embA IGR may increase EMB resistance through enhancement of the transcription of embA and embB, thus providing a novel mechanism of regulating the resistance level to EMB in M. tuberculosis clinical strains. In this study, the breakpoint of the MABA DST method was 1 μg/ml. When the MIC was more than 1 μg/ml, the strain was considered to be EMB resistant. Therefore, the MICs of some EMB-resistant strains by MGIT (Table 2) were less than 5 μg/ml, such as 2 μg/ml and 4 μg/ml. The MICs of four EMB-susceptible strains with embC-embA IGR mutations (−12C-T and −16C-T) judged by MGIT were more than 1 μg/ml. Thus, according to the results of MABA DST, higher transcriptional activity of these two mutant sites and higher binding affinity of embR, two strains with −12C-T and two strains with −16C-T should better be judged resistant strains.
The binding of EmbR to the embABC promoter region regulates transcription of the embCAB operon and increases the MIC to EMB (29). Our results showed that the binding activity of EmbR to mutant embC-embA IGR fragments was higher than that to the wild-type embC-embA IGR, suggesting that mutations in embC-embA IGR may facilitate the binding of EmbR to the promoter region of embAB to enhance embA and embB mRNA transcription, thus contributing to M. tuberculosis resistance to EMB. Whether embC-embA IGR mutations also increases the binding of additional transcriptional regulators to the embABC promoter region requires further investigation. Our data revealed that mutation of embC-embA IGRs increases its binding with phosphorylated EmbR (Fig. 5). We have also found that incubation of EmbR with PknH enhances its binding with either wild-type or embC-embA IGRs (data not shown). Thus, it appears that the binding of EmbR with embC-embA IGRs depends on two aspects: one is the phosphorylation state of EmbR, which could be induced by specific stimuli or autoactivation, and another is the specific sequence of embC-embA IGRs.
Molecular drug susceptibility testing based on embB mutations as a rapid method was used to test the drug susceptibility of M. tuberculosis to EMB. However, due to the low mutation rate of embB in EMB-resistant M. tuberculosis strains, the sensitivity of molecular drug susceptibility testing based on embB was too low to satisfy the needs of clinical diagnosis. According to our data, combining embC-embA IGR with embB as a diagnostic marker for drug susceptibility testing can increase sensitivity and NPV.
In summary, our data demonstrate that mutations in the embC-embA IGR contribute to M. tuberculosis resistance to EMB. These embC-embA IGR mutations could be used as a marker of molecular DST to enhance the sensitivity of molecular tests for drug resistance and also as an indicator of the drug resistance level in some clinical strains with embB mutations.
ACKNOWLEDGMENT
The National Natural Science Foundation of China supported this study (81201323).
Footnotes
Published ahead of print 2 September 2014
REFERENCES
- 1.Stop TB Department and Department of Child and Adolescent Health and Development of the World Health Organization. 2013. Ethambutol efficacy and toxicity: literature review and recommendations for daily and intermittent dosage in children. WHO/HTM/TB/2006.365. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.World Health Organization. 2013. Global tuberculosis report. WHO/HTM/TB/2013.11. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 3.Takayama K, Armstrong EL, Kunugi KA, Kilburn JO. 1979. Inhibition by ethambutol of mycolic acid transfer into the cell wall of Mycobacterium smegmatis. Antimicrob. Agents Chemother. 16:240–242. 10.1128/AAC.16.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu MH, Chiang CY, Deng YM, Wang TF, Jou R. 2013. Proficiency of drug susceptibility testing for Mycobacterium tuberculosis in Taiwan, 2007–2011. Int. J. Tuberc. Lung Dis. 17:113–119. 10.5588/ijtld.12.0521. [DOI] [PubMed] [Google Scholar]
- 5.Sangare L, Diande S, Kouanda S, Dingtoumda BI, Mourfou A, Ouedraogo F, Sawadogo I, Nebie B, Gueye A, Sawadogo LT, Traore AS. 2010. Mycobacterium tuberculosis drug-resistance in previously treated patients in Ouagadougou, Burkina Faso. Ann. Afr. Med. 9:15–19. 10.4103/1596-3519.62619. [DOI] [PubMed] [Google Scholar]
- 6.Sangaré L, Diandé S, Badoum G, Dingtoumda B, Traoré AS. 2010. Anti-tuberculosis drug resistance in new and previously treated pulmonary tuberculosis cases in Burkina Faso. Int. J. Tuberc. Lung Dis. 14:1424–1429. [PubMed] [Google Scholar]
- 7.Zhao Y, Xu S, Wang L, Chin DP, Wang S, Jiang G, Xia H, Zhou Y, Li Q, Ou X, Pang Y, Song Y, Zhao B, Zhang H, He G, Guo J, Wang Y. 2012. National survey of drug-resistant tuberculosis in China. N. Engl. J. Med. 366:2161–2170. 10.1056/NEJMoa1108789. [DOI] [PubMed] [Google Scholar]
- 8.Alcaide F, Pfyffer GE, Telenti A. 1997. Role of embB in natural and acquired resistance to ethambutol in mycobacteria. Antimicrob Agents Chemother. 41:2270–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Telenti A, Philipp WJ, Sreevatsan S, Bernasconi C, Stockbauer KE, Wieles B, Musser JM, Jacobs WR., Jr 1997. The emb operon, a unique gene cluster of Mycobacterium tuberculosis involved in resistance to ethambutol. Nat Med. 3:567–570. 10.1038/nm0597-567. [DOI] [PubMed] [Google Scholar]
- 10.Ramaswamy SV, Amin AG, Göksel S, Stager CE, Dou SJ, El Sahly H, Moghazeh SL, Kreiswirth BN, Musser JM. 2000. Molecular genetic analysis of nucleotide polymorphisms associated with ethambutol resistance in human isolates of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 44:326–336. 10.1128/AAC.44.2.326-336.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sreevatsan S, Stockbauer KE, Pan X, Kreiswirth BN, Moghazeh SL, Jacobs WR, Jr, Telenti A, Musser JM. 1997. Ethambutol resistance in Mycobacterium tuberculosis: critical role of embB mutations. Antimicrob Agents Chemother. 41:1677–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi D, Li L, Zhao Y, Jia Q, Li H, Coulter C, Jin Q, Zhu G. 2011. Characteristics of embB mutations in multidrug-resistant Mycobacterium tuberculosis isolates in Henan, China. J. Antimicrob Chemother. 66:2240–2247. 10.1093/jac/dkr284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramaswamy SV, Dou SJ, Rendon A, Yang Z, Cave MD, Graviss EA. 2004. Genotypic analysis of multidrug-resistant Mycobacterium tuberculosis isolates from Monterrey, Mexico. J. Med. Microbiol. 53:107–113. 10.1099/jmm.0.05343-0. [DOI] [PubMed] [Google Scholar]
- 14.Parsons LM, Salfinger M, Clobridge A, Dormandy J, Mirabello L, Polletta VL, Sanic A, Sinyavskiy O, Larsen SC, Driscoll J, Zickas G, Taber HW. 2005. Phenotypic and molecular characterization of Mycobacterium tuberculosis isolates resistant to both isoniazid and ethambutol. Antimicrob. Agents Chemother. 49:2218–2225. 10.1128/AAC.49.6.2218-2225.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Safi H, Sayers B, Hazbón MH, Alland D. 2008. Transfer of embB codon 306 mutations into clinical Mycobacterium tuberculosis strains alters susceptibility to ethambutol, isoniazid, and rifampin. Antimicrob. Agents Chemother. 52:2027–2034. 10.1128/AAC.01486-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Safi H, Fleischmann RD, Peterson SN, Jones MB, Jarrahi B, Alland D. 2010. Allelic exchange and mutant selection demonstrate that common clinical embCAB gene mutations only modestly increase resistance to ethambutol in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 54:103–108. 10.1128/AAC.01288-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Li D, Zhao L, Fleming J, Lin N, Wang T, Liu Z, Li C, Galwey N, Deng J, Zhou Y, Zhu Y, Gao Y, Wang T, Wang S, Huang Y, Wang M, Zhong Q, Zhou L, Chen T, Zhou J, Yang R, Zhu G, Hang H, Zhang J, Li F, Wan K, Wang J, Zhang XE, Bi L. 2013. Genome sequencing of 161 Mycobacterium tuberculosis isolates from China identifies genes and intergenic regions associated with drug resistance. Nat. Genet. 45:1255–1260. 10.1038/ng.2735. [DOI] [PubMed] [Google Scholar]
- 18.Thierry D, Cave MD, Eisenach KD, Crawford JT, Bates JH, Gicquel B, Guesdon JL. 1990. IS6110, an IS-like element of Mycobacterium tuberculosis complex. Nucleic Acids Res. 18:188. 10.1093/nar/18.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garrigó M, Aragón LM, Alcaide F, Borrell S, Cardeñosa E, Galán JJ, Gonzalez-Martín J, Martin-Casabona N, Moreno C, Salvado M, Coll P. 2007. Multicenter laboratory evaluation of the MB/BacT Mycobacterium detection system and the BACTEC MGIT 960 system in comparison with the BACTEC 460TB system for susceptibility testing of Mycobacterium tuberculosis. J. Clin. Microbiol. 45:1766–1770. 10.1128/JCM.02162-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franzblau SG, Witzig RS, McLaughlin JC, Torres P, Madico G, Hernandez A, Degnan MT, Cook MB, Quenzer VK, Ferguson RM, Gilman RH. 1998. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J. Clin. Microbiol. 36:362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desjardin LE, Perkins MD, Teixeira L, Cave MD, Eisenach KD. 1996. Alkaline decontamination of sputum specimens adversely affects stability of mycobacterial mRNA. J. Clin. Microbiol. 34:2435–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan XY, Ma H, Guo J, Li ZM, Cheng ZH, Guo SQ, Zhao GP. 2009. A novel differential expression system for gene modulation in mycobacteria. Plasmid 61:39–46. 10.1016/j.plasmid.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Molle V, Kremer L, Girard-Blanc C, Besra GS, Cozzone AJ, Prost JF. 2003. An FHA phosphoprotein recognition domain mediates protein EmbR phosphorylation by PknH, a Ser/Thr protein kinase from Mycobacterium tuberculosis. Biochemistry 42:15300–15309. 10.1021/bi035150b. [DOI] [PubMed] [Google Scholar]
- 24.Sharma K, Gupta M, Pathak M, Gupta N, Koul A, Sarangi S, Baweja R, Singh Y. 2006. Transcriptional control of the mycobacterial embCAB operon by PknH through a regulatory protein, EmbR, in vivo. J. Bacteriol. 188:2936–2944. 10.1128/JB.188.8.2936-2944.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaber AA, Ahmad S, Mokaddas E. 2009. Minor contribution of mutations at iniA codon 501 and embC-embA intergenic region in ethambutol-resistant clinical Mycobacterium tuberculosis isolates in Kuwait. Ann. Clin. Microbiol. Antimicrob. 8:2. 10.1186/1476-0711-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plinke C, Cox HS, Zarkua N, Karimovich HA, Braker K, Diel R, Rüsch-Gerdes S, Feuerriegel S, Niemann S. 2010. embCAB sequence variation among ethambutol-resistant Mycobacterium tuberculosis isolates without embB306 mutation. J. Antimicrob Chemother. 65:1359–13567. 10.1093/jac/dkq120. [DOI] [PubMed] [Google Scholar]
- 27.Belanger AE, Besra GS, Ford ME, Mikusová K, Belisle JT, Brennan PJ, Inamine JM. 1996. The embAB genes of Mycobacterium avium encode an arabinosyl transferase involved in cell wall arabinan biosynthesis that is the target for the antimycobacterial drug ethambutol. Proc. Natl. Acad. Sci. U S A. 93:11919–11924. 10.1073/pnas.93.21.11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amin AG, Goude R, Shi L, Zhang J, Chatterjee D, Parish T. 2008. EmbA is an essential arabinosyltransferase in Mycobacterium tuberculosis. Microbiology 154:240–248. 10.1099/mic.0.2007/012153-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Safi H, Lingaraju S, Amin A, Kim S, Jones M, Holmes M, McNeil M, Peterson SN, Chatterjee D, Fleischmann R, Alland D. 2013. Evolution of high-level ethambutol-resistant tuberculosis through interacting mutations in decaprenylphosphoryl-β-d-arabinose biosynthetic and utilization pathway genes. Nat Genet. 45:1190–1197. 10.1038/ng.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]


