Abstract
Leishmaniasis, a complex of diseases caused by protozoa of the genus Leishmania, is endemic in 98 countries, affecting approximately 12 million people worldwide. Current treatments for leishmaniasis have many disadvantages, such as toxicity, high costs, and prolonged treatment, making the development of new treatment alternatives highly relevant. Several studies have verified the antileishmanial activity of β-carboline compounds. In the present study, we investigated the in vitro antileishmanial activity of N-butyl-[1-(4-methoxy)phenyl-9H-β-carboline]-3-carboxamide (β-CB) against Leishmania amazonensis. The compound was active against promastigote, axenic amastigote, and intracellular amastigote forms of L. amazonensis, exhibiting high selectivity for the parasite. Moreover, β-CB did not exhibit hemolytic or mutagenic potential. Promastigotes treated with the alkaloid presented rounding of the body cell, cell membrane projections, an increase in the number of promastigotes presenting two flagella, and parasites of abnormal phenotype, with three or more flagella and/or nuclei. Furthermore, we observed an increase in the subpopulation of cells in the G2/M stage of the cell cycle. Altogether, these results suggest that β-CB likely prevents cytokinesis, although it does not interfere with the duplication of cell structures. We also verified an increase in O2·− production and the accumulation of lipid storage bodies. Cell membrane integrity was maintained, in addition to the absence of phosphatidylserine externalization, DNA fragmentation, and autophagosomes. Although the possibility of an apoptotic process cannot be discarded, β-CB likely exerts its antileishmanial activity through a cytostatic effect, thus preventing cellular proliferation.
INTRODUCTION
Leishmaniasis is a complex of infectious diseases caused by protozoa of the genus Leishmania. A total of 98 countries on five continents have reported endemic leishmaniasis transmission, with an overall prevalence of 12 million cases. More than 58,000 cases of visceral leishmaniasis and 220,000 cases of cutaneous leishmaniasis are officially registered each year. However, it is estimated that approximately 0.2 to 0.4 million visceral leishmaniasis cases and 0.7 to 1.2 million cutaneous leishmaniasis cases occur each year. These diseases are responsible for approximately 20,000 to 40,000 deaths annually (1, 2).
Leishmaniasis is classified according to a spectrum of clinical manifestations into cutaneous, diffuse cutaneous, mucocutaneous, and visceral leishmaniasis. In South America, the species L. amazonensis is one of the etiological agents of cutaneous leishmaniasis, which may progress to diffuse cutaneous leishmaniasis in immunosuppressed individuals (3).
Currently, the treatment of leishmaniasis mainly includes pentavalent antimonials, such as sodium stibogluconate and meglumine antimoniate, although these drugs present several disadvantages, such as toxicity, high costs, prolonged treatment, and parenteral or intralesional routes of administration. Moreover, these treatments are not always effective. As second-line drugs, amphotericin B and pentamidine are used, despite high toxicity. To reduce this toxicity, lipid and liposomal formulations of amphotericin B have been developed. Recently, the oral administration of miltefosine has been used for the treatment of visceral leishmaniasis in some countries, although this drug shows teratogenic potential (3–5).
Considering the drawbacks of the available treatments, several studies have been performed to develop new strategies for leishmaniasis treatment. β-Carboline alkaloids have drawn attention because of their biological activities against parasites of the Trypanosomatidae family, including antitrypanosomal activity (6–10) and antileishmanial activity (8, 11–13). A recent study demonstrated the antileishmanial and antitrypanosomal activity of a series of N-alkyl-(1-phenyl-substituted-tetrahydro-β-carboline)-3-carboxamides, showing that compounds containing N-butylcarboxamide groups were the most active, suggesting that these groups may improve the biological activity of these compounds (8). In the present study, we assessed the antileishmanial activity of the β-carboline compound N-butyl-[1-(4-methoxy)phenyl-9H-β-carboline]-3-carboxamide (β-CB) against different forms of L. amazonensis. We also investigated the morphological and biochemical alterations induced by this alkaloid.
MATERIALS AND METHODS
Chemicals.
Dimethyl sulfoxide (DMSO), folic acid, hemin, Schneider's medium, thiazolyl blue tetrazolium bromide (MTT), actinomycin D, antimycin A, carbonyl cyanide m-chlorophenylhydrazone (CCCP), Nile red, and monodansylcadaverine (MDC) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Brain heart infusion (BHI) was acquired from Beckon Dickinson (Sparks, MD, USA). Fetal bovine serum (FBS), RPMI 1640, and Giemsa were obtained from Invitrogen (Grand Island, NY, USA). May-Grunwald was purchased from Newprov (Pinhais, PR, Brazil). Annexin V fluorescein isothiocyanate (FITC) conjugate, 3,8-phenanthridine diamine-5-(6-triphenylphosphonium hexyl)-5,6-dihydro-6- phenyl (MitoSOX), and the APO bromodeoxyuridine (BrdU) terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay kit, including propidium iodide/RNase A (PI-RNase A), were obtained from Invitrogen (Eugene, OR, USA). Triton X-100 was obtained from Vetec (Rio de Janeiro, RJ, Brazil). All of the other reagents were of analytical grade.
Synthesis of β-CB compound.
β-CB was synthesized as previously described by Tonin et al. (8) and dissolved in DMSO before use. The molecular structure of the compound is shown in Fig. 1.
FIG 1.
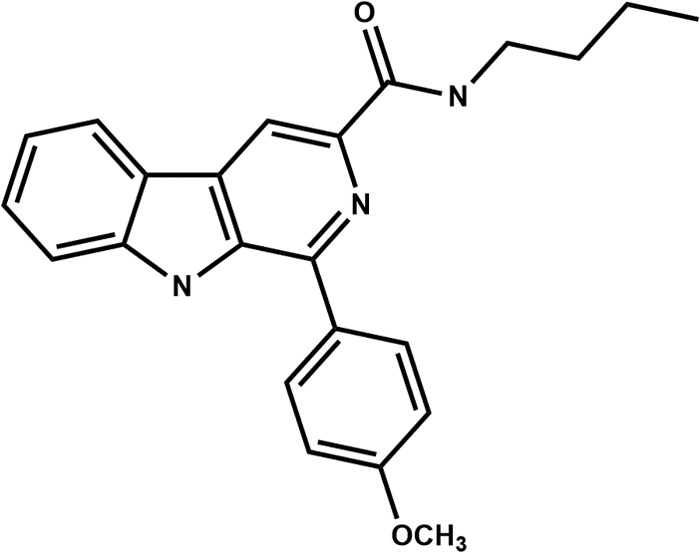
Molecular structure of the compound N-butyl-[1-(4-methoxy)phenyl-9H-β-carboline]-3-carboxamide.
Parasites and J774A1 macrophages.
L. amazonensis strain WHOM/BR/75/Josefa was originally isolated from a human case of diffuse cutaneous leishmaniasis by C. A. Cuba Cuba (Universidade de Brasília, DF, Brazil). Promastigote forms were cultured in Warren's medium supplemented with 10% heat-inactivated FBS at 25°C. Axenic amastigote forms were obtained by in vitro transformation (14) and cultured in Schneider's insect medium (pH 4.6) supplemented with 20% FBS at 32°C. J774A1 murine macrophages were maintained in RPMI 1640 medium with sodium bicarbonate and l-glutamine (pH 7.2), supplemented with 10% FBS at 37°C in a 5% CO2-air mixture.
Animals.
The animal protocols were approved by the ethics committee of the Universidade Estadual de Maringá (approval no. 038/2011). Male and female Swiss and BALB/c mice (8 to 12 weeks old) were housed in acrylic cages (20 by 35 by 13 cm) for at least 5 days before beginning the experiments. Throughout the adaptation and experimental periods, the mice were kept under controlled temperature (22 ± 1°C) and humidity conditions and in a 12-h/12-h light/dark cycle. The mice received water and Nuvilab CR1 Quimtia commercial diet chow (Colombo, PR, Brazil) ad libitum.
Antiproliferative assay.
Promastigote forms in the logarithmic phase were cultured (1 × 106 parasites/ml) in 24-well plates that contained Warren's medium supplemented with 10% FBS in the presence or absence of different concentrations of β-CB and incubated at 25°C for 72 h. For the axenic amastigote forms, the experiments were performed using Schneider's insect medium with the addition of 20% FBS in 12-well plates incubated at 32°C. The cell density of each treatment was verified by counting in a Neubauer chamber. The concentrations of β-CB that inhibited 50% and 90% of parasite growth (IC50 and IC90, respectively) were calculated.
Interaction between L. amazonensis and intraperitoneal macrophages.
Macrophages from the intraperitoneal cavity of BALB/c mice were harvested by washing with cold phosphate-buffered saline (PBS) with the addition of 3% FBS. After concentrating the cells by centrifugation at 4°C, the cells were plated (5 × 105 macrophages/ml) in RPMI 1640 medium supplemented with 10% FBS in 24-well plates with rounded coverslips and allowed to adhere for 2 h at 37°C in a 5% CO2 atmosphere. The plates were washed with culture medium to remove nonadherent cells and then infected with metacyclic promastigote forms (3.5 × 106 parasites/ml) to evaluate macrophage-parasite interactions. After 4 h, the cells were treated with different concentrations of β-CB for 48 h. The cells were washed in PBS, fixed in methanol for 10 min, and stained with 10% Giemsa for 40 min. To determine the percentage of infected cells and the number of parasites per cell, 200 macrophages were counted using an optical microscope: survival index (%) = number of parasites/macrophage × number of infected macrophages/200. The IC50 and IC90 values were calculated.
Cytotoxicity assay.
J774A1 macrophages, obtained from confluent cultures, were cultured at 5 × 105 cells/ml in RPMI 1640 medium supplemented with 10% FBS in 96-well microplates at 37°C in a 5% CO2 atmosphere. After 24 h, the cells were treated with different concentrations of β-CB and incubated for 48 h. The microplates were then washed with PBS, and 50 μl of MTT solution (2 mg/ml) was added to each well. The MTT assay is based on the conversion of water-soluble MTT to an insoluble formazan precipitate by viable mitochondria, which was allowed to occur for 4 h while protected from light. The precipitate was solubilized in 150 μl DMSO, and absorbance was read in a Bio-Tek Power Wave XS spectrophotometer at 492 nm. The cytotoxicity concentration for 50% of the cells (CC50) was determined. The selectivity index (SI) was calculated as CC50/IC50.
Hemolytic activity.
Human blood type A+ from a healthy volunteer donor was collected without anticoagulant and immediately defibrinated by contact with glass. The erythrocytes were harvested by centrifugation and washed in glycosylated saline (0.9% NaCl, 1% glucose) to remove any hemoglobin from eventual hemolysis until the supernatant was completely clear. The cells were inoculated in 96-well plates at 3% in glycosylated saline and treated with different concentrations of β-CB for 3 h at 37°C. Triton X-100 (1%) was used as a positive control. After centrifugation, the absorbance of the supernatant was evaluated in a Bio-Tek Power Wave XS spectrophotometer at 540 nm to estimate hemolysis: hemolysis (%) = (As − Ac) × 100/Ap, where As, Ac, and Ap are the absorbances of the test sample, negative control, and positive control, respectively.
Evaluation of mutagenicity by micronucleus assay.
Swiss mice were divided into three groups of 10 animals each (5 males, 5 females): the control group (orally treated by gavage with 0.9% NaCl saline solution), the positive-control group (orally treated with 40 mg/kg cyclophosphamide), and the β-CB group (orally treated with 400 mg/kg β-CB). Twenty-four hours postadministration, the animals were sacrificed, and the femurs were exposed to remove bone marrow by gently flushing with FBS. The samples were concentrated by centrifugation, and the cells were smeared on glass slides. The bone marrow smears were stained with May-Grunwald and Giemsa, and 2,000 polychromatic erythrocytes (PEs) were evaluated for the presence of a micronucleus using optical microscopy.
Scanning electron microscopy.
Promastigote forms were treated for 72 h as previously described for the antiproliferative assay using β-CB concentrations that corresponded to the IC50 and IC90. After incubation, the parasites were fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer at 4°C for 12 h. The cells were then adhered onto poly-l-lysine-coated coverslips, dehydrated in crescent grades of ethanol, critical-point dried with CO2, sputter coated with gold, and observed in a Shimadzu SS-550 scanning electron microscope. Parasite-macrophage interactions were also analyzed by scanning electron microscopy (SEM). The promastigote forms and macrophages were allowed to interact for 24 h before treatment with the compound (IC50 and IC90). All of the sample processing was performed on the coverslips.
Transmission electron microscopy.
Promastigote forms were treated for 72 h and fixed as described previously for SEM. The parasites were then postfixed with 1% OsO4, 0.8% potassium ferrocyanide, and 10 mM CaCl2 in 0.1 M cacodylate buffer at room temperature for 60 min. The cells were then dehydrated in crescent grades of acetone and embedded in Polybed 812 resin. Ultrathin sections were made and stained with uranyl acetate and lead citrate. The sections were observed in a JEOL JM 1400 transmission electron microscope.
Flagellum evaluation.
Promastigote forms were treated for 72 h as previously described for the antiproliferative assay using β-CB concentrations that corresponded to the IC50 and IC90. The cells were harvested and resuspended in FBS. The cells were smeared on glass slides and stained with May-Grunwald and Giemsa. The number of flagella in 250 parasites was counted by immersion optical microscopy.
Cell cycle.
Promastigote forms (5 × 106 parasites/ml) were treated with 2.7, 26.8, 133.9, and 267.8 μM β-CB for 24 h. After incubation, the cells were fixed in 70% cold methanol at 4°C for 1 h. Afterward, the parasites were washed in PBS, and 10 μl IP-RNase A was added, followed by incubation at 4°C for 45 min. Data acquisition and analysis were performed using a FACSCalibur flow cytometer equipped with CellQuest software. A total of 10,000 events were acquired in the region that corresponded to the parasites. The percentages of cells in each stage of the cell cycle were determined.
DNA fragmentation by TUNEL.
Promastigote forms were treated for 72 h as previously described for the antiproliferative assay using β-CB concentrations that corresponded to the IC50 and IC90. As a positive control, 10 μg/ml actinomycin D was used. The cells were fixed in 1% paraformaldehyde for 30 min in a cold bath. The samples were processed using the APO BrdU TUNEL assay kit according to the manufacturer's instructions. DNA double-strand ruptures were evaluated under an Olympus BX51 epifluorescence microscope equipped with an FITC filter. The images were recorded using an Olympus UC30 camera.
Lipid bodies assessed by Nile red accumulation.
Promastigote forms were treated for 72 h as previously described for the antiproliferative assay using β-CB concentrations that corresponded to the IC50 and IC90. The cells were incubated in 10 μg/ml Nile red for 30 min at room temperature. The accumulation of Nile red on lipid bodies in the parasites was observed under an Olympus BX51 epifluorescence microscope. The images were recorded with an Olympus UC30 camera.
Detection of mitochondrion-derived O2·− by MitoSOX.
Promastigote forms were washed twice with Krebs-Henseleit buffer (15 mM NaHCO3, 5 mM KCl, 120 mM NaCl, 0.7 mM Na2HPO4·2H2O, and 1.5 mM NaH2PO4·2H2O; pH 7.3) and incubated in 5 μM MitoSOX for 10 min at room temperature. Afterward, the parasites (2 × 107 cells/ml) were treated in 96-well microplates with 13.4, 26.8, 133.9, and 267.8 μM β-CB. As a positive control, 10 μM antimycin A, a known stimulus that induces O2·− production by mitochondria, was used. The fluorescence of oxidized MitoSOX was measured by fluorimetry on a PerkinElmer Victor X3, with an excitation wavelength of 510 nm and an emission wavelength of 580 nm, immediately and 1, 2, and 3 h posttreatment.
Cell membrane integrity and phosphatidylserine exposure.
Promastigote forms (5 × 106 parasites/ml) were treated with 2.7, 26.8, 133.9, and 267.8 μM β-CB for 24 h. CCCP (100 μM) was used as a positive control. The cells were harvested, washed twice in PBS, and resuspended in 100 μl binding buffer (140 mM NaCl, 5 mM CaCl2, and 10 mM HEPES-Na, pH 7.4), followed by the addition of 5 μl annexin-V FITC at room temperature for 15 min. After incubation, 400 μl of binding buffer and 0.2 μg/ml PI were added. Data acquisition and analysis were performed using a FACSCalibur flow cytometer equipped with CellQuest software. A total of 10,000 events were acquired in the region that corresponded to the parasites.
Evaluation of autophagic vacuoles by MDC.
Promastigote forms were treated for 72 h as previously described for the antiproliferative assay using β-CB concentrations that corresponded to the IC50 and IC90. The cells were incubated with 0.05 mM MDC for 1 h at room temperature. The parasites were then washed twice in PBS and analyzed for the presence of autophagic vacuoles using an Olympus BX51 epifluorescence microscope equipped with a DAPI (4′,6-diamidino-2-phenylindole) filter. The images were recorded with an Olympus UC30 camera.
Statistical analysis.
Data were obtained from at least three independent experiments and are expressed as means ± standard deviation (SD). The statistical analyses were performed using one- or two-way analysis of variance (ANOVA) followed by the Tukey post hoc test. P values of ≤0.05 were considered statistically significant. All the statistical analyses were performed using Prism 5 software (GraphPad, San Diego, CA, USA).
RESULTS
β-CB inhibits proliferation in vitro of L. amazonensis.
Antiproliferative assays demonstrated that β-CB has antileishmanial activity against promastigote and axenic amastigote forms of L. amazonensis in a concentration-dependent manner. For promastigotes, which occur in the alimentary tract of insect vectors, the IC50 and IC90 values were 4.44 ± 0.36 µM and 12.14 ± 0.56 μM, respectively. For axenic amastigotes, which are present in mammalian hosts and were axenically cultivated herein, the IC50 and IC90 values were 12.51 ± 0.62 µM and 24.29 ± 2.28 μM, respectively. β-CB was also evaluated against amastigotes internalized in intraperitoneal murine macrophages, with IC50 and IC90 values of 14.51 ± 3.7 µM and 29.21 ± 7.36 μM, respectively.
Cytotoxicity to mammalian cells.
The cytotoxicity of β-CB was evaluated in J774A1 macrophages. The cells were treated with different concentrations of the alkaloid for 48 h, and the CC50 was 249.38 ± 35.32 μM. The SIs for promastigotes, axenic amastigotes, and intracellular amastigotes were 56.2, 20.0, and 17.2, respectively, demonstrating that the compound is highly selective for the parasites, independent of the evolutive form analyzed. The ability of β-CB to lyse erythrocytes was also assessed. The maximum concentration tested was 267.8 μM, which induced only 3.53% hemolysis (Fig. 2). To evaluate if β-CB has mutagenic potential, mice were orally treated with 400 mg/kg β-CB. The mice did not show an increase in the number of micronucleated polychromatic erythrocytes (MPEs) in bone marrow after 24 h of administration (13.8 ± 8.3 MPEs/2,000 PEs) compared to the negative control (9 ± 3.8 MPEs/2,000 PEs). The positive control, 40 mg/kg cyclophosphamide, increased the presence of these cells in bone marrow (21.6 ± 9.4 MPEs/2,000 PEs) (Fig. 3).
FIG 2.
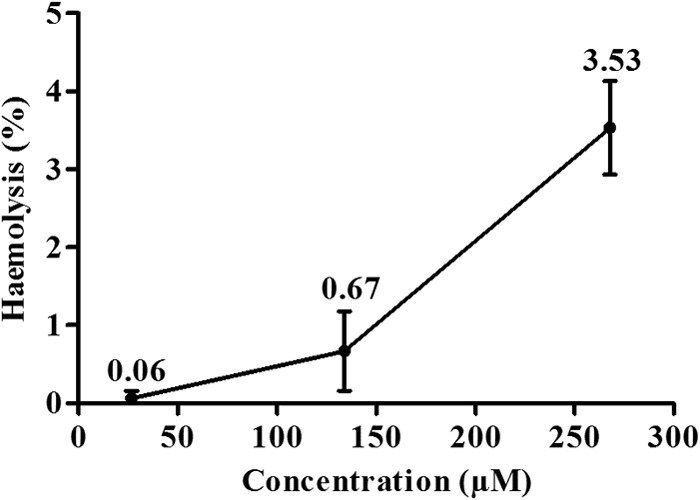
Hemolytic activity of N-butyl-[1-(4-methoxy)phenyl-9H-β-carboline]-3-carboxamide. The data are expressed as means ± SD from at least three independent experiments.
FIG 3.
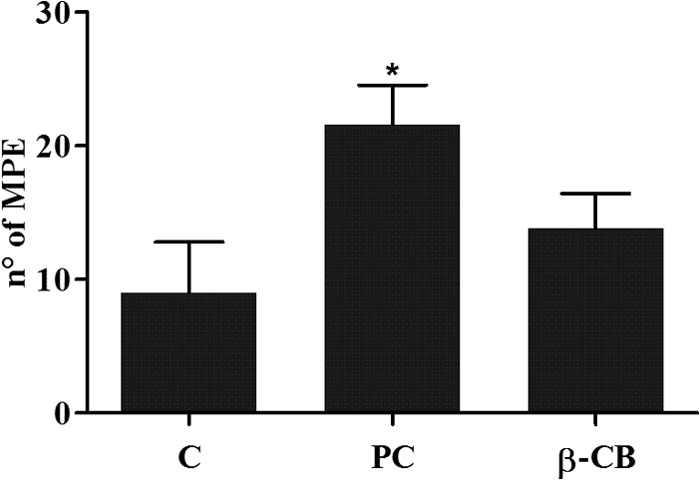
Frequency of micronucleated polychromatic erythrocytes (MPEs) in bone marrow of Swiss mice orally treated with 400 mg/kg N-butyl-[1-(4-methoxy)phenyl-9H-β-carboline]-3-carboxamide (β-CB) or 40 mg/kg cyclophosphamide (positive control [PC]) after 24 h of administration (n = 10; 5 males/5 females). Two thousand polychromatic erythrocytes were counted per animal. The data are expressed as means ± SD. One-way ANOVA followed by Tukey post hoc test. *, P ≤ 0.05 compared to negative control (C).
Promastigotes treated with β-CB present multiple nuclei and flagella.
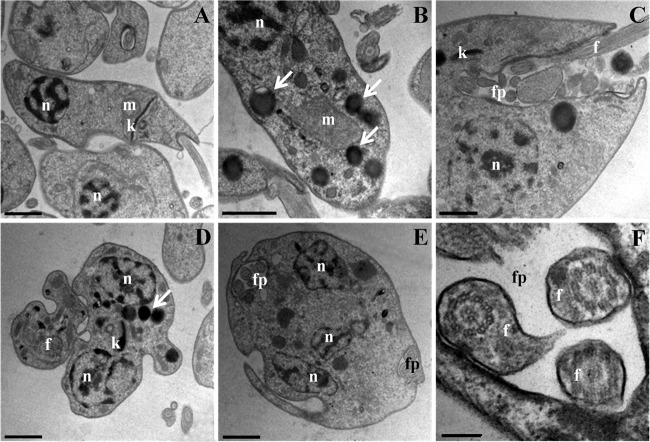
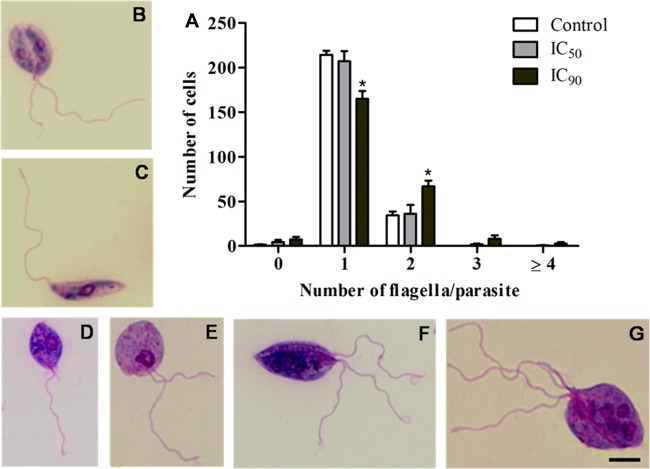
SEM analysis revealed that promastigote forms treated with the IC50 and IC90 of β-CB presented rounded cell bodies, cell membrane projections, and multiple flagella (Fig. 4). Similar alterations (except flagella) were observed in intracellular amastigote forms treated with β-CB, including damage to the cell membrane that was likely related to an interaction between the alkaloid and macrophages (Fig. 5). These findings were confirmed by transmission electron microscopy (TEM) analysis (Fig. 6), in which multiple flagella and nuclei could be visualized (Fig. 6E). In Fig. 6F, a flagellum cross-section shows normal axoneme architecture, forming a ring of nine outer microtubule doublets associated with dynein arms, around a pair of central microtubules. Cell body projections are seen in Fig. 6D and E. Figure 6B and C show the marked presence of lipid storage bodies and vacuolization into a flagellar pocket, respectively, for promastigotes treated with the IC50 of β-CB. Optical microscopy analyses demonstrated that promastigotes treated with the IC90 of β-CB had a reduction in the number of parasites with one flagellum and an increase in the number of cells with two structures. Additionally, parasites with three or more flagella, which were not seen in the control cells, were found in β-CB-treated cultures. Cells with multiple nuclei and kinetoplasts were also visualized by this method (Fig. 7).
FIG 4.
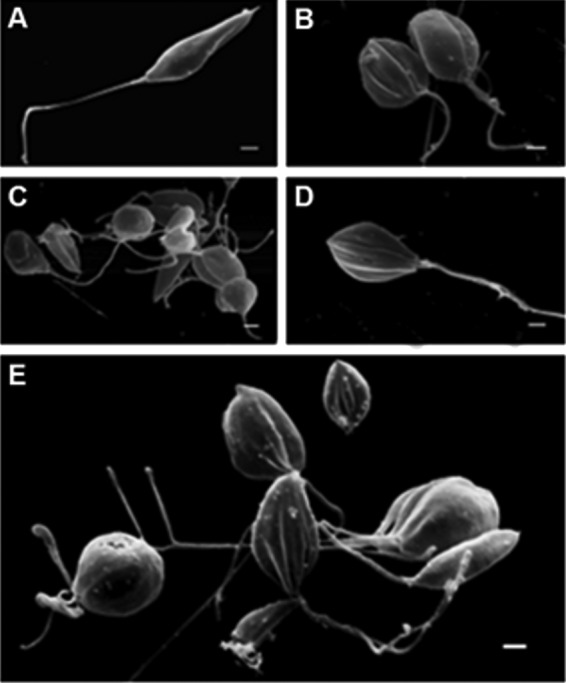
Morphological alterations in promastigote forms of Leishmania amazonensis treated with N-butyl-[1-(4-methoxy)phenyl-9H-β-carboline]-3-carboxamide (β-CB) for 72 h, visualized by scanning electron microscopy (SEM). (A) Control cells; (B, C) IC50 β-CB (4.44 μM); (D, E) IC90 β-CB (12.14 μM). Bars, 1 μm.
FIG 5.
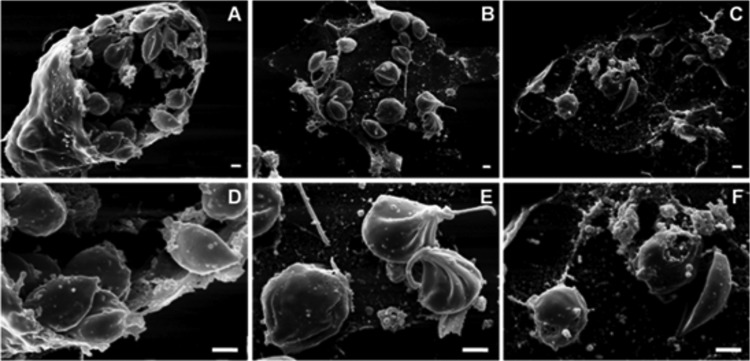
Morphological alterations in intracellular amastigote forms of Leishmania amazonensis treated with N-butyl-[1-(4-methoxy)phenyl-9H-β-carboline]-3-carboxamide (β-CB) for 24 h, visualized by scanning electron microscopy (SEM). (A, D) Control cells; (B, E) IC50 β-CB (14.51 μM); (C, F) IC90 β-CB (29.21 μM). Bars, 1 μm.
FIG 6.
Ultrastructural alterations in promastigote forms of Leishmania amazonensis treated with N-butyl-[1-(4-methoxy)phenyl-9H-β-carboline]-3-carboxamide (β-CB) for 72 h, visualized by transmission electron microscopy (TEM). (A) Control cells; (B, C) IC50 β-CB (4.44 μM); (D to F) IC90 β-CB (12.14 μM). n, nucleus; k, kinetoplast; fp, flagellar pocket; f, flagellum; m, mitochondrion; arrows, lipid-storage bodies. Bars, 1 μm (A through E) and 0.2 μm (F).
FIG 7.
(A) Number of flagella in promastigote forms of Leishmania amazonensis treated with N-butyl-[1-(4-methoxy)phenyl-9H-β-carboline]-3-carboxamide (β-CB) for 72 h and stained with May-Grunwald and Giemsa. (B, C) Control cells; (D, E) IC50 of β-CB (4.44 μM); (F, G) IC90 of β-CB (12.14 μM). The data are expressed as means ± SD from at least three independent experiments. Two-way ANOVA followed by Tukey post hoc test. *, P ≤ 0.05 compared to control. Bars, 5 μM.
Cell cycle arrest at the G2/M phase.
Cell cycle analysis by flow cytometry indicated that promastigotes treated with β-CB in crescent concentrations (2.7 to 133.9 μM) presented a decrease in the subpopulation of cells in the G0/G1 phase and an increase in the subpopulation of cells in the G2/M phase. Although not significant, a tendency toward an increase in the population of cells in the super-G2 phase was verified (Fig. 8). Furthermore, the sub-G0/G1 cell population (cells that undergo fragmentation of nuclear chromatin by DNases, a typical feature of apoptosis [15]) was not increased by treatment with β-CB. The absence of cells with fragmented DNA was confirmed by the TUNEL assay (data not shown).
FIG 8.
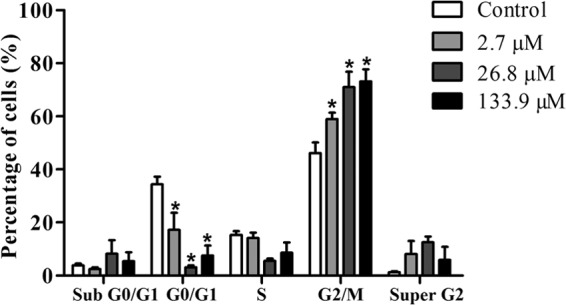
Cell cycle analysis of Leishmania amazonensis promastigote forms treated with N-butyl-[1-(4-methoxy)phenyl-9H-β-carboline]-3-carboxamide (β-CB) for 24 h evaluated by flow cytometry. The data are expressed as means ± SD from at least three independent experiments. Two-way ANOVA followed by Tukey post hoc test. *, P ≤ 0.05 compared to control.
β-CB induces an increase in lipid storage bodies and O2·− production in promastigotes.
Because transmission electron microscopy analysis of promastigotes treated with β-CB demonstrated the presence of vacuoles, suggesting the accumulation of lipid storage bodies (Fig. 6), a Nile red assay was performed by fluorescence microscopy, confirming an increase in the presence of lipid bodies in cells treated with the IC50 and IC90 of β-CB (Fig. 9). We also evaluated the mitochondrial production of O2·− in promastigotes treated with crescent concentrations of β-CB (13.4 to 267.8 μM) (Fig. 10). All of the tested concentrations increased the production of this reactive oxygen species (ROS) after 1 h of treatment. The higher concentrations (26.8 to 267.8 μM) induced an immediate elevation in O2·− concentrations.
FIG 9.
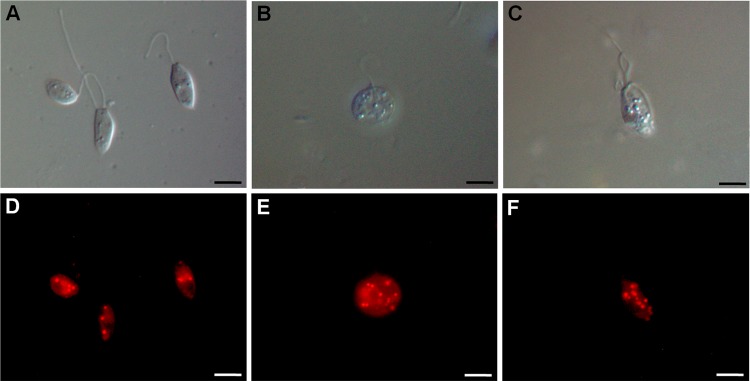
Lipid storage bodies demonstrated by Nile red accumulation in promastigote forms of Leishmania amazonensis treated with N-butyl-[1-(4-methoxy)phenyl-9H-β-carboline]-3-carboxamide (β-CB) for 72 h. Upper images were obtained by differential interference contrast (DIC) microscopy and lower images by fluorescence microscopy. (A, D) Control cells; (B, E) IC50 of β-CB (4.44 μM); (C, F) IC90 of β-CB (12.14 μM). Bars, 5 μm.
FIG 10.
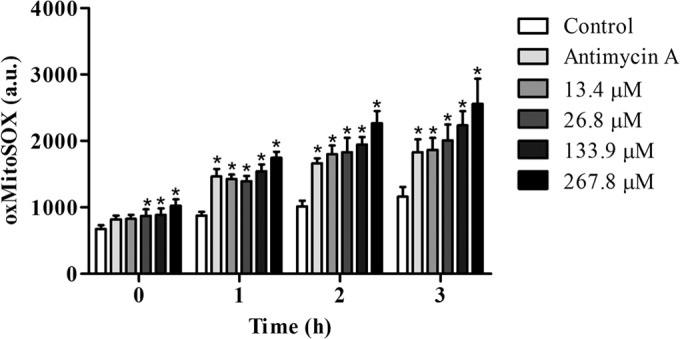
Mitochondrial O2·− production in promastigote forms of Leishmania amazonensis treated with N-butyl-[1-(4-methoxy)phenyl-9H-β-carboline]-3-carboxamide (β-CB) for up to 3 h, evaluated by the MitoSOX assay. Oxidized MitoSOX was measured in arbitrary units (a.u.). The data are expressed as means ± SD from at least three independent experiments. Two-way ANOVA followed by Tukey post hoc test. *, P ≤ 0.05 compared to control.
β-CB does not induce externalization of phosphatidylserine, disruption of the plasma membrane, and exacerbated autophagic process.
To corroborate the hypothesis that β-CB prevents cytokinesis in L. amazonensis as a cytostatic and not a cytotoxic effect, certain alterations that characterize different cell death pathways were assessed, including externalization of phosphatidylserine in apoptotic cells, disruption of the plasma membrane in necrotic cells, and the presence of high numbers of autophagic vacuoles in cells that are suffering cell death by autophagy (15–18). We performed flow cytometric analyses using PI labeling to identify cell membrane disruption and annexin V FITC labeling to investigate the externalization of phosphatidylserine. Treatment with crescent concentrations of β-CB (2.7 to 267.8 μM) did not induce an increase in the percentage of cells with cell membrane damage or extracellular exposition of phosphatidylserine (data not shown). We used MDC as a lysosomotropic autofluorescent marker of autophagic vacuoles, through an ion-trapping mechanism and interaction with lipid molecules as a solvent polarity probe (19). Autophagic vacuoles were not observed in promastigotes treated with the IC50 and IC90 of β-CB (data not shown).
DISCUSSION
Although many efforts have been made to improve the treatment of leishmaniasis, the currently available drugs and strategies still have many disadvantages, such as toxic effects, growing rates of resistance, high costs, and prolonged treatment periods. Several studies have sought to develop new treatment alternatives for leishmaniasis. β-Carboline compounds, characterized by a tricyclic pyrido[3,4-b]indole ring structure, have been evaluated as potential antileishmanial molecules (8, 11, 12). In the present study, we evaluated the activity of the β-carboline compound β-CB against L. amazonensis, which causes cutaneous and diffuse cutaneous forms of leishmaniasis. In a previous study, this compound was shown to be the most active of a series of N-alkyl-(1-phenyl-substituted-tertrahydro-β-carboline)-3-carboxamides against promastigotes of L. amazonensis (8), justifying its choice for this study. In the present study, it was also active against axenic and intracellular amastigote forms, exhibiting high selectivity for the parasite, independent of the evolutive form assessed. Furthermore, β-CB presented more pronounced antileishmanial activity than another β-carboline compound presenting the N-butylcarboxamide group, N-butyl-1-(4-dimethylamino)phenyl-1,2,3,4-tetrahydro-β-carboline-3-carboxamide, against the same species and evolutive form (11). However, its activity was slightly lower than a similar compound possessing an N-benzylcarboxamide in place of the N-butylcarboxamide group (12).
It is known that some drugs used for the treatment of leishmaniasis, such as amphotericin B and miltefosine, have high hemolytic activity. Although this makes them unfeasible for intravenous drug administration, it does not prevent their use (20, 21). β-CB did not present a significant hemolytic potential at the highest concentration tested. To corroborate its safety, its mutagenic potential was studied using the micronucleus assay. Micronuclei are chromatin-containing bodies that arise from whole chromosomes or chromosome fragments that are not incorporated into daughter nuclei following mitosis, and their high presence indicates genotoxicity. The alkaloid did not induce an increase in MPEs in bone marrow 24 h after β-CB administration, thus not presenting mutagenic potential in the evaluated conditions. Altogether, these results demonstrate that β-CB has promising antileishmanial activity associated with low toxicity and the absence of mutagenicity, which translates to high selectivity for the Leishmania parasite.
In order to determine the potential targets of β-CB in the parasite, morphological and ultrastructural alterations were studied by electron and optical microscopy. Rounding of body cells, cell membrane projections, and parasites with three or more flagella and nuclei were verified. These findings were also observed by Pedroso et al. (12) in promastigotes of L. amazonensis treated with a β-carboline compound of similar structure, as previously mentioned. During the cell cycle of Leishmania, nuclear and kinetoplast DNA duplication occurs simultaneously (S phase). New flagellum growth begins during the S phase and becomes visible outside the flagellar pocket near the end of this phase of the cell cycle; segregation of nuclear DNA occurs during the anaphase of mitosis, closely followed by kinetoplast division and cytokinesis, which is initiated at the anterior part of the parasite between the two flagella. However, differences in the order and timing of cell cycle events among Leishmania species have been described (22–24). Our findings indicate that, although the alkaloid β-CB did not prevent the duplication of flagella, kinetoplasts, or nuclei, cytokinesis was impaired, resulting in abnormal phenotypes of the parasite. Corroborating these results, the cell cycle analysis demonstrated that β-CB treatment induced an increase in the subpopulation of promastigotes in the G2/M phase (DNA duplicated) and a tendency toward an increase in the population of cells in the super-G2 phase (abnormal phenotypes), confirming the deleterious effects on cytokinesis with no influence on the duplication of genetic material. L. donovani promastigotes treated with three different antimicrotubule agents, ansamitocin P3, paclitaxel, and hemiasterlin, had the same alterations in cell cycle (25). Microtubules, assembled from α- and β-tubulin heterodimers, are related to several cellular events, such as cell shape and polarity, endocytosis, locomotion (formation of flagellum axoneme), nuclear division (mitotic spindle), and cytokinesis. In trypanosomatids, a subpellicular corset of cytoplasmic microtubules which are cross-linked to plasma membrane is found (26–30). In our case, β-CB did not impair new flagellum growth or modify its structural organization arrangement, segregation of nuclear DNA, or kinetoplast division. It seemed to act only on cytokinesis, which suggests two possibilities: β-CB antileishmanial activity is not due to an antimicrotubule mechanism of action, or it acts specifically on cytokinesis-related microtubule events. The latter hypothesis has been considered by other authors that verified abnormal phenotypes on L. amazonensis promastigotes treated with other substances (31, 32). Furthermore, it is necessary to consider that, although cytokinesis is primarily a microtubule-mediated process, actin dynamics is required to trigger the initiation of furrow formation, including basal body separation and flagellar pocket division (33); therefore, actin cannot be discarded as a cellular target of β-CB.
As observed by TEM and Nile red fluorescent labeling, β-CB induced an increase in lipid-storage bodies. The Nile red dye is not specific to a particular lipid (e.g., fatty acids, ergosterol, and phospholipids), and determining which lipid underwent alterations in its metabolic pathways is not possible using this method. Moreover, verifying whether the alterations occurred through lipid synthesis, utilization, or degradation is also not possible. Lipid body formation can be caused by different perturbations to the parasite's cellular functions. Rodrigues et al. (32) described the presence of many lipid droplets with distinct morphologies in the cytoplasm of L. amazonensis promastigotes treated with two squalene synthase (enzyme of the sterol biosynthesis pathway) inhibitors, attributing these findings to accumulation of lipid precursors due to alterations in the sterol content of the parasite's membranes. Abundant lipid bodies were also reported by Godinho et al. (34) in L. amazonensis promastigotes treated with an alkyl phosphocholine-dinitroaniline hybrid molecule, which the authors suggest is related to alterations in the phospholipid and sterol content of the parasite. Lipid accumulations were verified in epimastigotes of Trypanosoma cruzi treated with paclitaxel, an antimicrotubule agent (26), demonstrating that perturbations in the parasite not primarily related to lipid biosynthesis can induce lipid body formation. It is also necessary to consider that β-CB may be interacting with phospholipids of plasma and mitochondrial membranes due to its hydrophobic character, and, in this case, this action may be related to the impairment of cytokinesis, once microtubules are cross-linked to the plasma membrane, as mentioned above.
The existence of a single mitochondrion in parasites of the Trypanosomatidae family and its implication in ATP synthesis and redox homeostasis make this organelle an interesting target for antiprotozoal drugs. Some studies have shown that β-carboline compounds can induce mitochondrial dysfunction in trypanosomatids (10, 11, 35). Valdez et al. (10) observed that T. cruzi epimastigotes treated with N-butyl-1-(4-dimethylamino)phenyl-1,2,3,4-tetrahydro-β-carboline-3-carboxamide presented mitochondrial swelling. Volpato et al. (11) reported that the same compound induced depolarization of the mitochondrial membrane potential and an increase in the formation of mitochondrial O2·− in L. amazonensis promastigotes. Rivas et al. (35) attributed the activity of β-carboline (9H-pyrido-[3,4-b]-indole) alkaloids against T. cruzi epimastigotes to respiratory chain inhibition. Considering that mitochondrial dysfunction may be either a cause or a consequence of deregulation on the redox balance, leading to oxidation of cellular constituents, such as proteins, lipids, and DNA (36, 37), we evaluated the mitochondrial production of O2·− in promastigotes treated with β-CB, verifying an increase on O2·− concentrations. During oxidative phosphorylation by a mitochondrial electron transport chain, electrons can be transferred directly to oxygen molecules, generating O2·−, which may result in the generation of other reactive oxygen species, such as H2O2 and OH−, and consequently cellular damage (37, 38).
To exert an antiproliferative effect, antiprotozoal agents need to induce morphological, biochemical, or molecular alterations that lead to the loss of cellular function (parasite death) or prevent its proliferation. Cell death pathways may be generally classified into apoptosis (i.e., programmed cell death characterized by cytoplasmic retraction, chromatin condensation, chromosomal DNA fragmentation, mitochondrial swelling, exposure of phosphatidylserine residues at the outer plasma membrane, and activation of caspases), necrosis (i.e., an unregulated process characterized by irreversible disruption of plasma membrane integrity, organelle breakdown, and randomly fragmented DNA), and exacerbation of the autophagic process, whereby cells remove or remodel damaged cell structures but under certain conditions can induce an intense cytoplasmic vacuolization and dismantling of the cellular organization, resulting in cell death (16–18). In order to determine the manner by which β-CB exerted its antiproliferative activity, we performed flow cytometry analyses using PI dye to identify cell membrane disruption (in necrotic cells) and annexin V FITC to investigate the externalization of phosphatidylserine, a typical feature of apoptotic cells (18). Treatment with crescent concentrations of β-CB did not alter the percentage of cells labeled with PI, demonstrating that the parasites did not suffer cell membrane damage and probably did not undergo a necrotic process. Also, β-CB treatment did not increase the externalization of phosphatidylserine in plasma membrane of promastigotes, as verified by annexin-V FITC labeling. This result, associated with an absence of DNA fragmentation verified by the TUNEL assay and cell cycle analysis, indicates that β-CB probably does not induce the cells to undergo apoptosis. However, the elevation of reactive oxygen species, such as the O2·− observed in this study, is associated with oxidative stress and an apoptotic process (39), although alterations in mitochondrial morphology were not verified. Finally, MDC labeling was performed to evaluate a possible autophagic programmed cell death in promastigotes treated with β-CB. Autophagic vacuoles were not observed by fluorescence microscopy, indicating that antiproliferative activity of β-CB likely does not result from an exacerbated autophagic process. Considering that the alkaloid induced the presence of many lipid droplets in the cytoplasm but an autophagic process that degraded this accumulation was not verified, the intense exocytic activity in the region of the flagellar pocket may be related to an attempt to remove these lipid deposits from the intracellular environment or even to remove other cellular components, since β-CB-treated parasites fail to complete the cytokinesis but not to duplicate genetic material, as previously discussed. Lee et al. (40) demonstrated that α-synuclein-expressing cells subjected to inhibition of autophagic function via both pharmacological and genetic methods presented increased exocytosis of this protein. The absence of classic features of different types of cell death (i.e., externalization of phosphatidylserine, cell membrane disruption, DNA fragmentation, and a pronounced autophagic process) in promastigotes of L. amazonensis treated with the compound corroborates the hypothesis that this alkaloid induces a cytostatic effect in the parasite by impairment of cytokinesis. However, promastigotes treated with β-CB presented many lipid storage bodies and increased O2·− production, as discussed above, which does not allow us to discard a cell death pathway.
Several studies, as reviewed by Cao et al. (41), have demonstrated that β-carboline compounds can interact with DNA, leading to alterations in the processes of DNA replication, repair, and cleavage. In this study, β-CB did not induce DNA fragmentation, cells with multiple nuclei were observed, and mutagenic potential was not verified; therefore, a possible interaction of β-CB with DNA is not clear.
In conclusion, our findings indicate that β-CB has activity against L. amazonensis and is highly selective for the parasite. Moreover, β-CB presents low mutagenic and hemolytic potential. This alkaloid likely impairs cytokinesis, thus preventing proliferation of the parasite by a cytostatic effect, although the possibility of an apoptotic process cannot be completely discarded. Future studies with this compound will promote a better understanding of its mechanism of action and contribute to the development of new antileishmanial drugs.
ACKNOWLEDGMENTS
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Capacitação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Financiadora de Estudos e Projetos (FINEP), Programa de Núcleos de Excelência (PRONEX/Fundação Araucária), Programa de Pós-Graduação em Ciências Biológicas da Universidade Estadual de Maringá, and Complexo de Centrais de Apoio a Pesquisa (COMCAP-UEM).
Footnotes
Published ahead of print 15 September 2014
REFERENCES
- 1.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, WHO Leishmaniasis Control Team 2012. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7:e35671. 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvar J, Yactayo S, Bern C. 2006. Leishmaniasis and poverty. Trends Parasitol. 22:552–557. 10.1016/j.pt.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Reithinger R, Dujardin J, Louzir H, Pirmez C, Alexander B, Brooker S. 2007. Cutaneous leishmaniasis. Lancet Infect. Dis. 7:581–596. 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 4.Tiuman TS, Santos AO, Ueda-Nakamura T, Dias Filho BP, Nakamura CV. 2011. Recent advances in leishmaniasis treatment. Int. J. Infect. Dis. 15:e525–e532. 10.1016/j.ijid.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Croft SL, Seifert K, Yardley V. 2006. Current scenario of drug development for leishmaniasis. Indian J. Med. Res. 123:399–410. [PubMed] [Google Scholar]
- 6.Valdez RH, Tonin LT, Ueda-Nakamura T, Silva SO, Dias Filho BP, Kaneshima EN, Yamada-Ogatta SF, Yamauchi LM, Sarragiotto MH, Nakamura CV. 2012. In vitro and in vivo trypanocidal synergistic activity of N-butyl-1-(4-dimethylamino)phenyl-1,2,3,4-tetrahydro-β-carboline-3-carboxamide associated with benzonidazole. Antimicrob. Agents Chemother. 56:507–512. 10.1128/AAC.05575-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costa EV, Pinheiro ML, de Souza AD, Barison A, Campos FR, Valdez RH, Ueda-Nakamura T, Filho BP, Nakamura CV. 2011. Trypanocidal activity of oxoaporphine and pyrimidine-β-carboline alkaloids from the branches of Annona foetida Mart. (Annonaceae). Molecules 16:9714–9720. 10.3390/molecules16119714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tonin LT, Panice MR, Nakamura CV, Rocha KJ, dos Santos AO, Ueda-Nakamura T, da Costa WF, Sarragiotto MH. 2010. Antitrypanosomal and antileishmanial activities of novel N-alkyl-(1-phenylsubstituted-beta-carboline)-3-carboxamides. Biomed. Pharmacother. 64:386–389. 10.1016/j.biopha.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Tonin LT, Barbosa VA, Bocca CC, Ramos ER, Nakamura CV, da Costa WF, Basso EA, Nakamura TU, Sarragiotto MH. 2009. Comparative study of the trypanocidal activity of the methyl 1-nitrophenyl-1,2,3,4–9H-tetrahydro-beta-carboline-3-carboxylate derivatives and benznidazole using theoretical calculations and cyclic voltammetry. Eur. J. Med. Chem. 44:1745–1750. 10.1016/j.ejmech.2008.03.044. [DOI] [PubMed] [Google Scholar]
- 10.Valdez RH, Tonin LT, Ueda-Nakamura T, Dias Filho BP, Morgado-Diaz JA, Sarragiotto MH, Nakamura CV. 2009. Biological activity of 1,2,3,4-tetrahydro-beta-carboline-3-carboxamides against Trypanosoma cruzi. Acta Trop. 110:7–14. 10.1016/j.actatropica.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Volpato H, Desolti VC, Cogo J, Panice MR, Sarragiotto MH, Silva SO, Ueda-Nakamura T, Nakamura CV. 2013. The effects of N-butyl-1-(4-dimethylamino)phenyl-1,2,3,4-tetrahydro-β-carboline-3-carboxamide against Leishmania amazonensis are mediated by mitochondrial dysfunction. Evid. Based Complement. Alternat. Med. 2013:874367. 10.1155/2013/874367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pedroso RB, Tonin LT, Ueda-Nakamura T, Dias Filho BP, Sarragiotto MH, Nakamura CV. 2011. Beta-carboline-3-carboxamide derivatives as promising antileishmanial agents. Ann. Trop. Med. Parasitol. 105:549–557. 10.1179/2047773211Y.0000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Giorgio C, Delmas F, Ollivier E, Elias R, Balansard G, Timon-David P. 2004. In vitro activity of the beta-carboline alkaloids harmane, harmine and harmaline toward parasites of the species Leishmania infantum. Exp. Parasitol. 106:67–74. 10.1016/j.exppara.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Ueda-Nakamura T, Attias M, Souza W. 2001. Megasome biogenesis in Leishmania amazonensis: a morphometric and cytochemical study. Parasitol. Res. 87:89–97. 10.1007/s004360000319. [DOI] [PubMed] [Google Scholar]
- 15.Kaczanowski S, Mohammed S, Reece SE. 2011. Evolution of apoptosis-like programmed cell death in unicellular protozoan parasites. Parasit. Vectors 4:44. 10.1186/1756-3305-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adade CM, Chagas GSF, Souto-Padrón T. 2012. Apis mellifera venom induces different cell death pathways in Trypanosoma cruzi. Parasitology 139:1444–1461. 10.1017/S0031182012000790. [DOI] [PubMed] [Google Scholar]
- 17.Guimarães C, Linden R. 2004. Programmed cell deaths: apoptosis and alternative deathstyles. Eur. J. Biochem. 271:1638–1650. 10.1111/j.1432-1033.2004.04084.x. [DOI] [PubMed] [Google Scholar]
- 18.Debrabant A, Nakhasi H. 2003. Programmed cell death in trypanosomatids: is it an altruistic mechanism for survival of the fittest? Kinetoplastid Biol. Dis. 2:7. 10.1186/1475-9292-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niemann A, Takatsuki A, Elsasser HP. 2000. The lysosomotropic agent monodansylcadaverine also acts as a solvent polarity probe. J. Histochem. Cytochem. 48:251–258. 10.1177/002215540004800210. [DOI] [PubMed] [Google Scholar]
- 20.Munoz C, Alzoubi K, Jacobi J, Abed M, Lang F. 2013. Effect of miltefosine on erythrocytes. Toxicol. In Vitro 27:1913–1919. 10.1016/j.tiv.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Izumi E, Ueda-Nakamura T, Veiga VF, Jr, Pinto AC, Nakamura CV. 2012. Terpenes from Copaifera demonstrated in vitro antiparasitic and synergic activity. J. Med. Chem. 12:2994–3001. 10.1021/jm201451h. [DOI] [PubMed] [Google Scholar]
- 22.da Silva MS, Monteiro JP, Nunes VS, Vasconcelos EJ, Perez AM, Freitas-Junior LH, Elias MC, Canol MIN. 2013. Leishmania amazonensis promastigotes present two distinct modes of nucleus and kinetoplast segregation during cell cycle. PLoS One 8:e81397. 10.1371/journal.pone.0081397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ambit A, Woods KL, Cull B, Coombs GH, Mottram JC. 2011. Morphological events during the cell cycle of Leishmania major. Eukaryot. Cell 10:1429–1438. 10.1128/EC.05118-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wheeler RJ, Gluenz E, Gull K. 2011. The cell cycle of Leishmania: morphogenetic events and their implications for parasite biology. Mol. Microbiol. 79:647–662. 10.1111/j.1365-2958.2010.07479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Havens CG, Bryant N, Asher L, Lamoreaux L, Perfetto S, Brendle JJ, Werbovetz KA. 2000. Cellular effects of leishmanial tubulin inhibitors on L. donovani. Mol. Biochem. Parasitol. 110:223–236. 10.1016/S0166-6851(00)00272-3. [DOI] [PubMed] [Google Scholar]
- 26.Dantas AP, Barbosa HS, de Castro SL. 2003. Biological and ultrastructural effects of the anti-microtubule agent taxol against Trypanosoma cruzi. J. Submicrosc. Cytol. Pathol. 35:287–294. [PubMed] [Google Scholar]
- 27.Kohl L, Robinson D, Bastin P. 2003. Novel roles for the flagellum in cell morphogenesis and cytokinesis of trypanosomes. EMBO J. 22:5336–5346. 10.1093/emboj/cdg518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gull K. 2001. Protist tubulins: new arrivals, evolutionary relationships and insights to cytoskeletal function. Curr. Opin. Microbiol. 4:427–432. 10.1016/S1369-5274(00)00230-7. [DOI] [PubMed] [Google Scholar]
- 29.Gull K. 1999. The cytoskeleton of trypanosomatid parasites. Annu. Rev. Microbiol. 53:629–655. 10.1146/annurev.micro.53.1.629. [DOI] [PubMed] [Google Scholar]
- 30.Ploubidou A, Robinson DR, Docherty RC, Ogbadoyi EO, Gull K. 1999. Evidence for novel cell cycle checkpoints in trypanosomes: kinetoplast segregation and cytokinesis in the absence of mitosis. J. Cell Sci. 112:4641–4650. [DOI] [PubMed] [Google Scholar]
- 31.Borges VM, Lopes UG, de Souza W, Vannier-Santos MA. 2005. Cell structure and cytokinesis alterations in multidrug-resistant Leishmania (Leishmania) amazonensis. Parasitol. Res. 95:90–96. 10.1007/s00436-004-1248-8. [DOI] [PubMed] [Google Scholar]
- 32.Rodrigues JCF, Concepcion JL, Rodrigues C, Caldera A, Urbina JA, Souza W. 2008. In vitro activities of ER-119884 and E5700, two potent squalene synthase inhibitors, against Leishmania amazonensis: antiproliferative, biochemical, and ultrastructural effects. Antimicrob. Agents Chemother. 52:4098–4114. 10.1128/AAC.01616-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tammana TV, Sahasrabuddhe AA, Bajpai VK, Gupta CM. 2010. ADF/cofilin-driven actin dynamics in early events of Leishmania cell division. J. Cell Sci. 123:1894–1901. 10.1242/jcs.068494. [DOI] [PubMed] [Google Scholar]
- 34.Godinho JLP, Georgikopoulou K, Calogeropoulou T, Souza W, Rodrigues JCF. 2013. A novel alkyl phosphocholine-dinitroaniline hybrid molecule exhibits biological activity in vitro against Leishmania amazonensis. Exp. Parasitol. 135:153–165. 10.1016/j.exppara.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Rivas P, Cassels BK, Morello A, Repetto Y. 1999. Effects of some beta-carboline alkaloids on intact Trypanosoma cruzi epomastigotes. Comp. Biochem. C Pharmacol. Toxicol. Endocrinol. 122:27–31. 10.1016/S0742-8413(98)10069-5. [DOI] [PubMed] [Google Scholar]
- 36.Ba X, Gupta S, Davidson M, Garg NJ. 2010. Trypanosoma cruzi induces the reactive oxygen species-PARP-1-RelA pathway for up-regulation of cytokine expression in cardiomyocytes. J. Biol. Chem. 285:11596–11606. 10.1074/jbc.M109.076984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mandelker L. 2008. Introduction to oxidative stress and mitochondrial dysfunction. Vet. Clin. North Am. Small Anim. Pract. 38:1–30. 10.1016/j.cvsm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Menna-Barreto RFS, Goncalves RLS, Costa EM, Silva RSF, Pinto AV, Oliveira MF, Catro SL. 2009. The effects on Trypanosoma cruzi of novel synthetic naphthoquinones are mediated by mitochondrial dysfunction. Free Radic. Biol. Med. 47:644–653. 10.1016/j.freeradbiomed.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Moreira W, Leprohon P, Quellette M. 2011. Tolerance to drug-induced cell death favours the acquisition of multidrug resistance in Leishmania. Cell Death Dis. 2:e201. 10.1038/cddis.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee HJ, Cho ED, Lee KW, Kim JH, Cho SG, Lee SJ. 2013. Autophagic failure promotes the exocytosis and intercellular transfer of α-synuclein. Exp. Mol. Med. 45:e22. 10.1038/emm.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao R, Peng W, Wang Z, Xu A. 2007. β-Carboline alkaloids: biochemical and pharmacological functions. Curr. Med. Chem. 14:479–500. 10.2174/092986707779940998. [DOI] [PubMed] [Google Scholar]