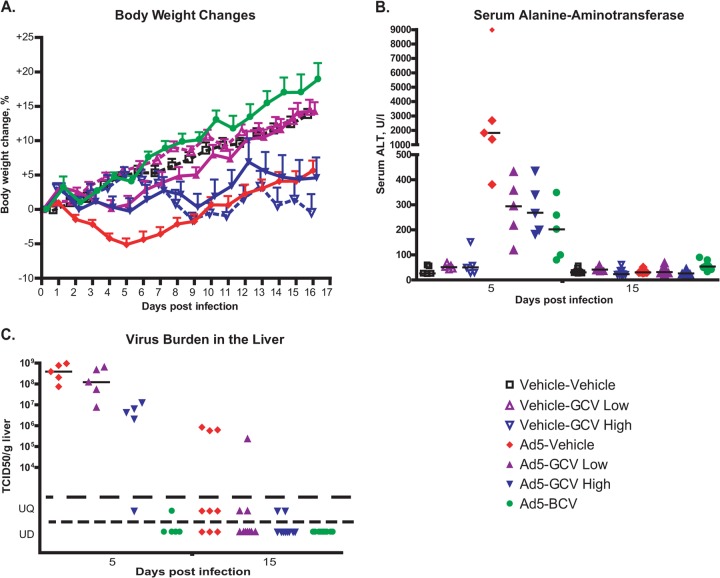
FIG 1.
GCV treatment reduces Ad5 replication and pathogenicity after intravenous challenge with the virus in immunosuppressed Syrian hamsters. (A) GCV reduces weight loss. Hamsters immunosuppressed with CP were injected with 1.5 × 1010 PFU of Ad5 i.v. on day 0 and treated with 33 mg/kg of GCV (GCV low) or 100 mg/kg of GCV (GCV high) daily. BCV was administered at a daily dose of 2.5 mg/kg. Drug treatment started at 12 h before virus challenge. Each symbol represents the group mean (calculated for the animals designated for the survival study; n = 10); the whiskers signify the standard errors of the means. For Ad5-vehicle versus Ad5-GCV low, P < 0.0001; for Ad5-vehicle versus Ad5-GCV high, P = 0.1650; and for Ad5-vehicle versus Ad5-BCV, P < 0.0001 (two-way ANOVA). (B) GCV treatment reduces liver pathogenicity. Each symbol represents an individual animal (n = 5 for day 5 and n = 9 or 10 for day 15); the horizontal bars signify the medians. For samples collected at 5 days postchallenge, for Ad-vehicle versus Ad5-GCV low, P = 0.0159; for Ad-vehicle versus Ad5-GCV high, P = 0.0159; and for Ad-vehicle versus Ad5-BCV, P = 0.0079 (Mann-Whitney U test). (C) GCV inhibits the replication of Ad5 in the liver. Each symbol represents the value from an individual animal (n = 5 for day 5 and n = 9 or 10 for day 15); the horizontal bars signify the means. For the day 5 samples, for Ad vehicle versus Ad5-GCV low, P = 0.3095; for Ad vehicle versus Ad5-GCV high, P < 0.01; and for Ad vehicle versus Ad5-BCV, P < 0.01 (Mann-Whitney U test). UQ, unquantifiable; UD, undetectable.