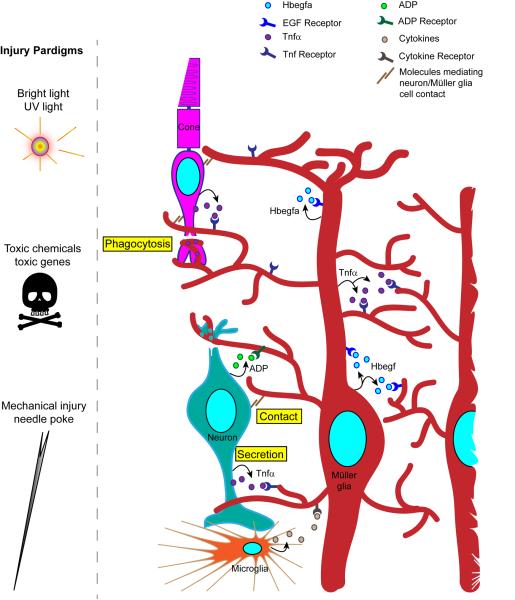
Figure 3. Injury paradigms and the communication of injury to Müller glia.
Various injury paradigms have been used to induce retinal damage and stimulate regeneration in zebrafish. These include prolonged exposure to intense bright light, short exposure to ultraviolet (UV) light; intravitreal injection of toxins (such as oubain and N-methyl-D-aspartate (NMDA)); expression of a toxic gene (such as bacterial nitroreductase, which, in combination with a prodrug generates a cytotoxic product); and mechanical injury (such as that resulting from a needle poke)11,12,69,81,82. Light-based damage paradigms generally destroy a population of photoreceptors, whereas toxins can cause wide-spread damage. Cytotoxic gene products can be directed to specific retinal cell types using appropriate promoters to drive their expression. Mechanical injury generally destroys all retinal cell types in a circumscribed region of the retina. The figure illustrates the ways in which injured cells might communicate with Müller glia to stimulate their reprogramming. These include secretion of signaling molecules (arrows) from damaged cells, Müller glia or infiltrating microglia; altered contact between damaged cells and Müller glia; and phagocytosis of injured cells by Müller glia. Recent studies have suggested that growth factors, such as heparin-binding epidermal growth factor (Hbegf), and cytokines, such as tumor necrosis factor- α (Tnfα), are necessary for Müller glia reprogramming and progenitor formation in the injured retina83,84. These factors are produced in Müller glia at the injury site and therefore, may act in an autocrine and paracrine fashion. Tnfα and ADP are also released from injured retinal neurons84,87.