Figure 3. α-NETA is safe and suppresses clinical EAE.
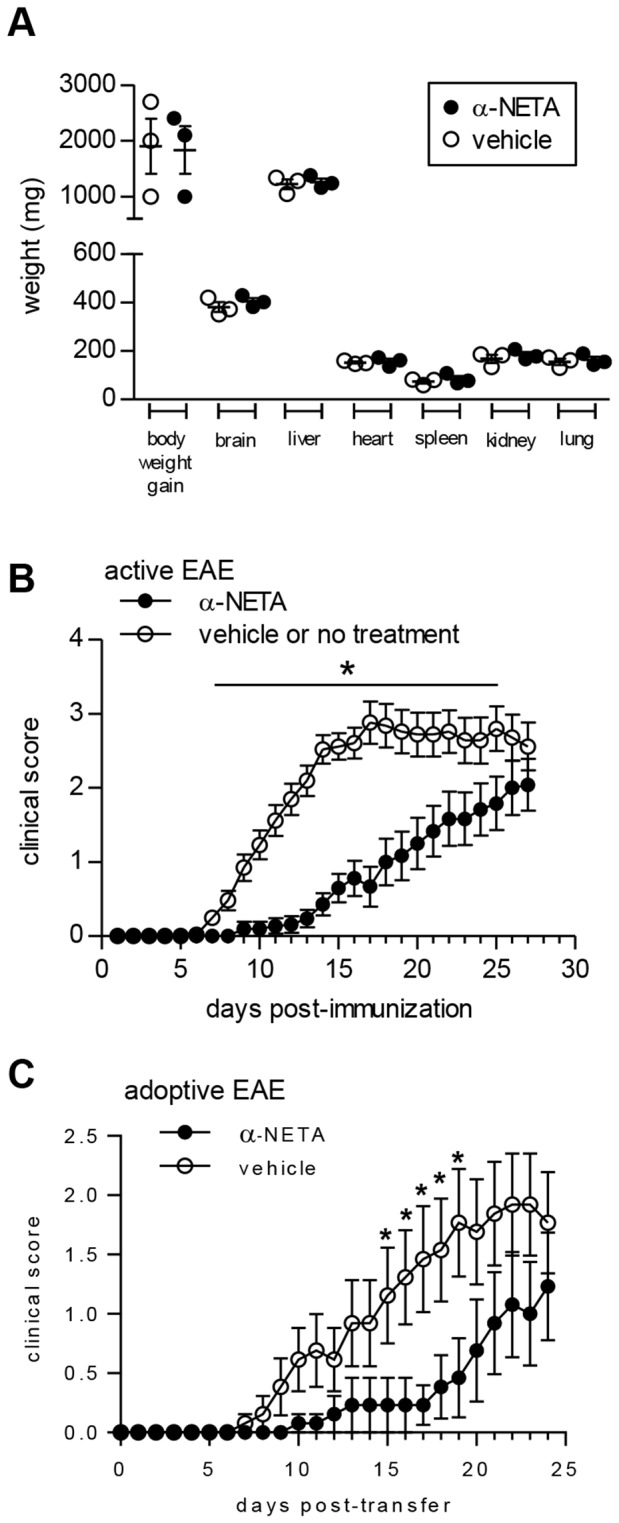
(A) C57BL/6 mice were injected s.c. with α-NETA (10 mg/kg) or vehicle daily for 16 days. The mice were then euthanized and vital organs weighed. Each symbol represents an individual mouse, and the bars indicate mean ± SEM, n = 3 mice per group. (B) EAE was induced in C57BL/6 mice by active immunization with MOG35-55/CFA. Mice received α-NETA (10 mg/kg daily, s.c. injection) beginning at the time of disease induction and were monitored daily for clinical disease as described in Materials and Methods. Control mice received either captisol vehicle or no treatment. The pooled data from five independent experiments with 7–10 mice per group is displayed, mean clinical score ± SEM. For days 1–15, n = 52 mice per group; for days 16–27, n = 24–25 mice per group. * p<0.05 by Mann-Whitney U test. (C) EAE was induced in C57BL/6 mice by passive transfer of MOG35–55-reactive lymphocytes derived from actively immunized EAE mice. Recipient mice received either vehicle or α-NETA (10 mg/kg) by daily s.c. injection beginning at the time of transfer and were scored daily for clinical disease. The pooled data from three independent experiments with 3–5 mice per group is displayed, mean clinical score ± SEM. * p<0.05 by Mann-Whitney U test.
