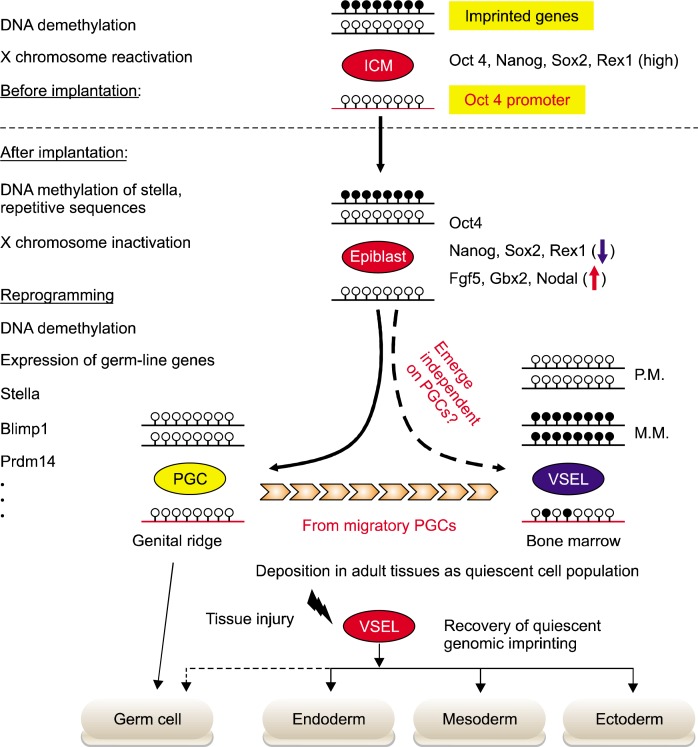
Fig. 1.
Epigenetic modification of VSELs during embryogenesis and tissue regeneration. Epigenetic modifications control the differentiation potential of stem cells during embryogenesis and tissue regeneration. During the implantation of embryo, ICM-derived epiblast stem cells methylated again i) X chromosome, ii) promoters for the genes characteristic for PSCs in ICM (Rex-1 and Stella), and iii) repetitive sequences. However, at the beginning of gastrulation the proximal, epiblast-specified PGCs can reset their epigenetic profile to one that characterizes ICM-derived PSCs. Subsequently, during PGCs migration to genital ridges, the global DNA demethylation leads to erase the genomic imprints. As it is hypothesized that VSELs originate from the epiblast-derived PGCs population, they show the PGCs-like epigenetic profiles, including the partial DNA demethylation in the regulatory DNA elements of several pluripotency, germ-line genes, and genomic imprints. The epigenetic profiles of developing VSELs are retained after their deposition into adult tissue. This parental-specific reprogramming of genomic imprinting of VSELs deposited in adult -tissue, e.g. BM, functions as i) a “lock-in mechanism” to prevent their unleashed proliferation and ii) a mechanism to restrict their sensitivity to Ins/Igf signaling. After exposure to tissue injury, quiescent VSELs de-repress “locked-in” genomic imprints along with progressive methylation of DNA in the Oct4 promoter. As a result of these epigenetic changes, VSELs become involved in the tissue regeneration process by differentiation into cells of all three germ layers, i.e. meso-, ecto-, and endoderm.