Abstract
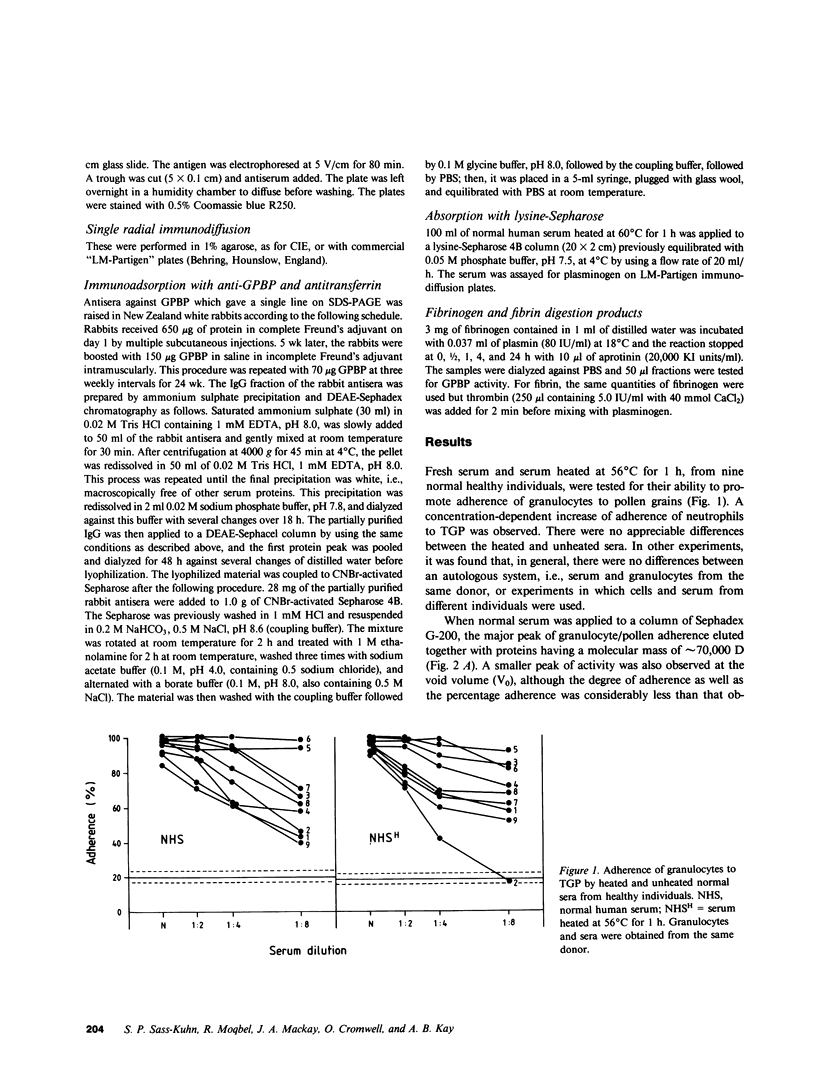
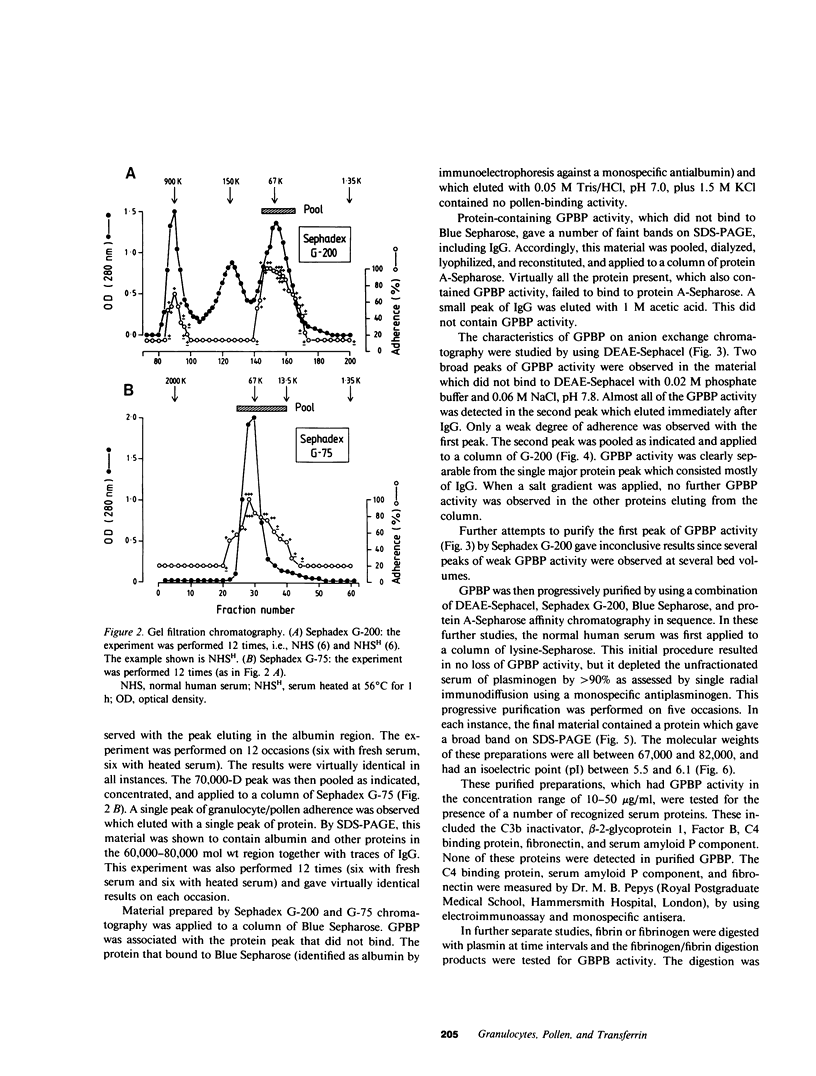
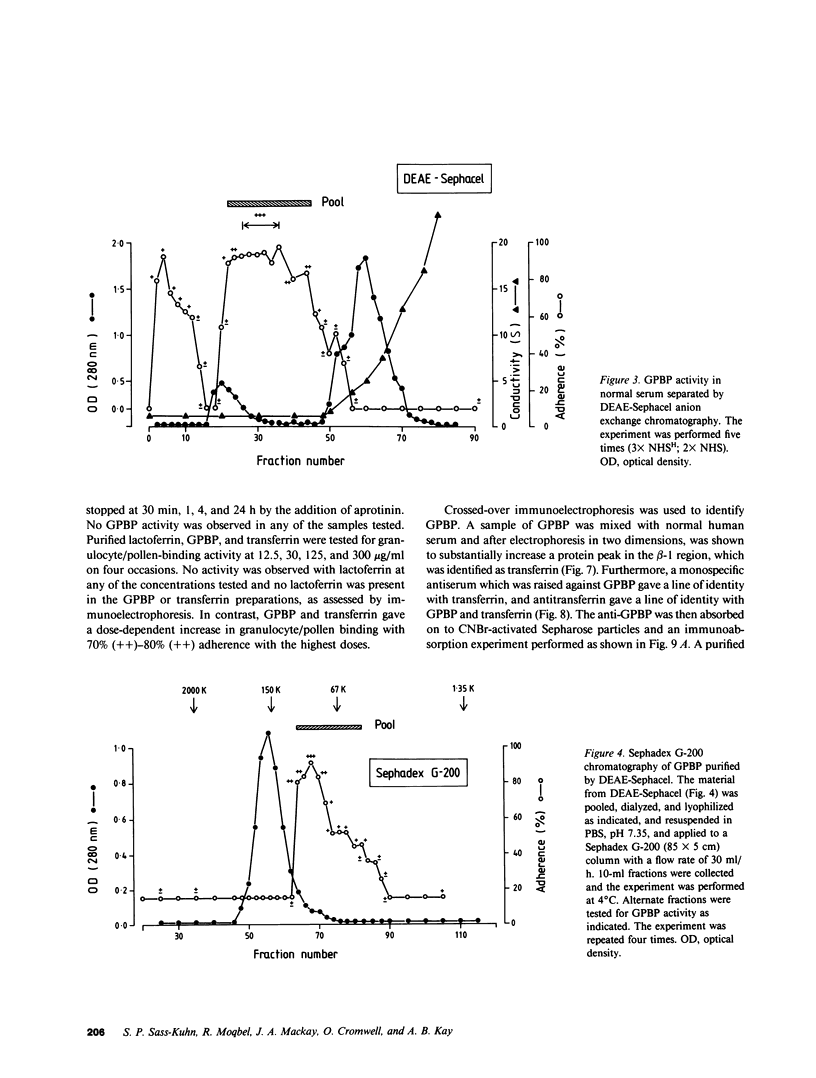
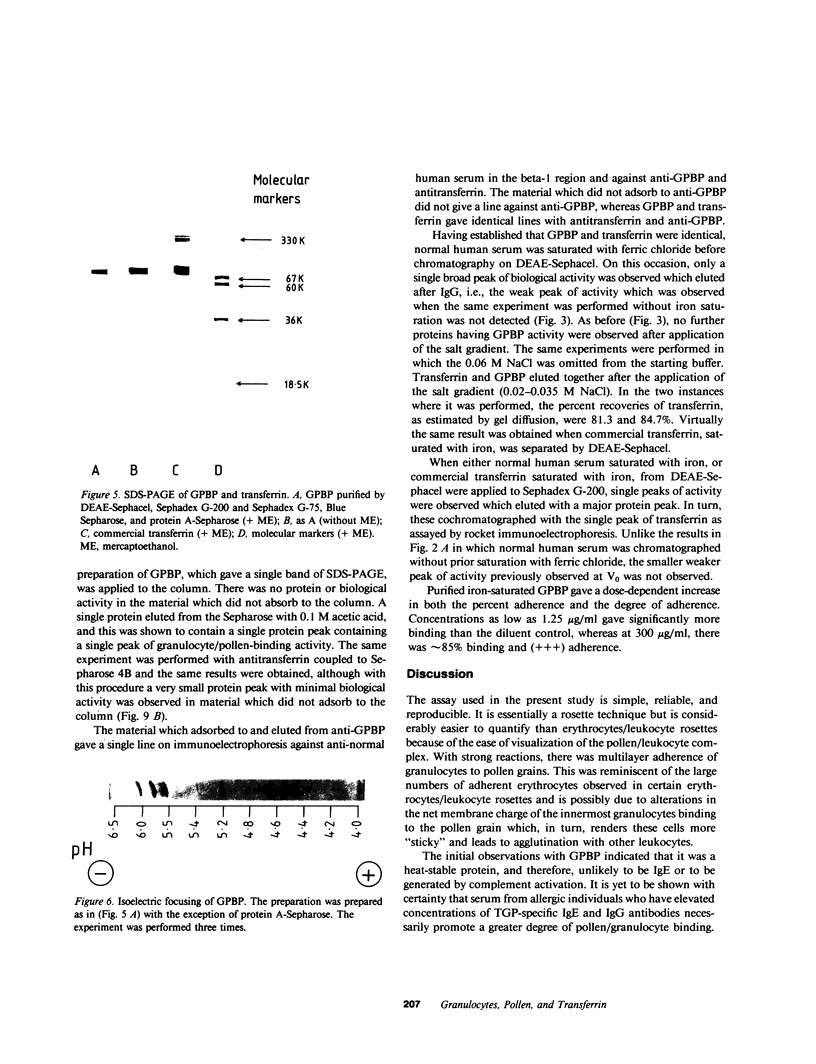
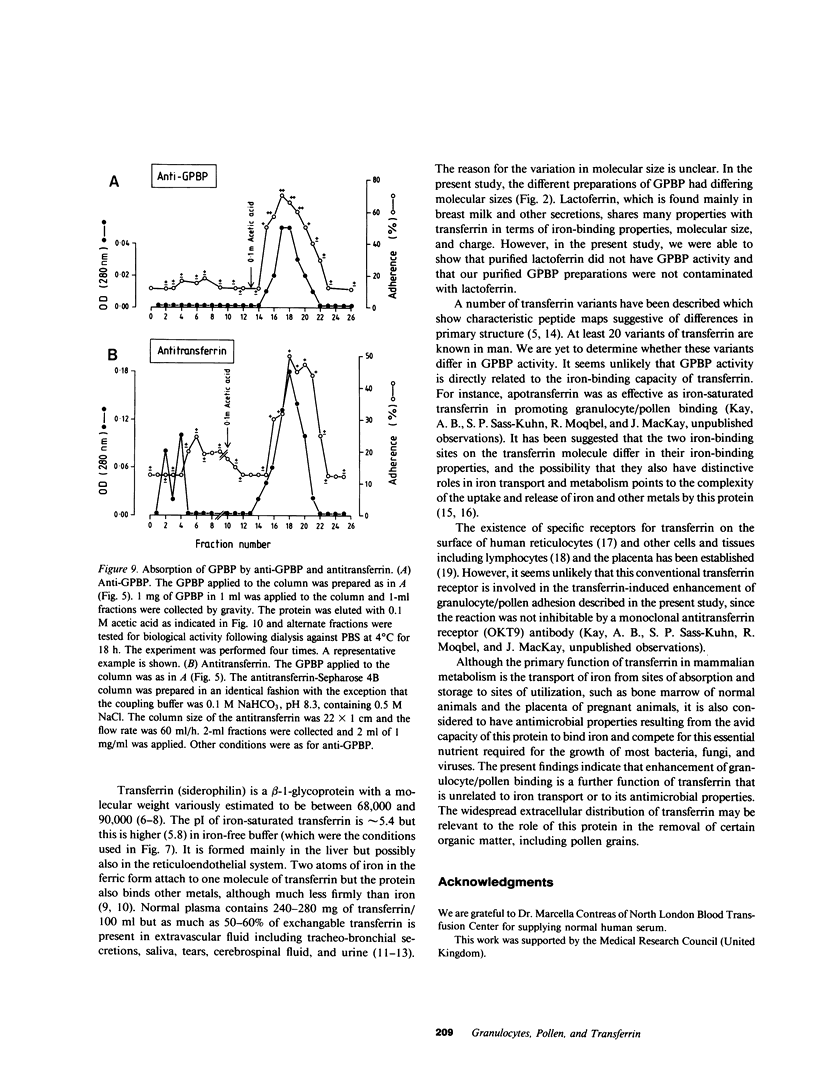
Normal human serum was found to contain a heat-stable protein which promoted the binding of granulocytes to timothy grass pollen (granulocyte/pollen-binding protein [GPBP]). GPBP was purified by gel filtration, anion exchange, and affinity chromatography. Virtually all of the granulocyte/pollen-binding activity was associated with a beta-1-protein having a molecular mass of approximately 77,000 D and an isoelectric point of between 5.5 and 6.1. By immunoelectrophoresis and sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the protein was identified as transferrin. Monospecific antisera raised against either GPBP or transferrin removed biological activity from GPBP preparations, and GPBP and transferrin gave lines of identity with these two antisera. The apparent heterogeneity in the molecular size and charge of GPBP observed during progressive purification was minimal when GPBP was saturated with ferric ions before the separation procedures. These experiments indicate that granulocyte/pollen binding is a hitherto unrecognized property of transferrin which appears to be unrelated to iron transport and raises the possibility that transferrin might have a physiological role in the removal of certain organic matter.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Aasa R., Redfield A. G. The chromium, manganese, and cobalt complexes of transferrin. J Biol Chem. 1969 Sep 10;244(17):4628–4633. [PubMed] [Google Scholar]
- Aisen P., Brown E. B. Structure and function of transferrin. Prog Hematol. 1975;9:25–56. [PubMed] [Google Scholar]
- Anwar A. R., Smithers S. R., Kay A. B. Killing of schistosomula of Schistosoma mansoni coated with antibody and/or complement by human leukocytes in vitro: requirement for complement in preferential killing by eosinophils. J Immunol. 1979 Feb;122(2):628–637. [PubMed] [Google Scholar]
- Bezkorovainy A., Grohlich D., Gerbeck C. M. Some physical-chemical properties of reduced-alkylated and sulphitolysed human serum transferrins and hen's-egg conalbumin. Biochem J. 1968 Dec;110(4):765–770. doi: 10.1042/bj1100765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAUSEN J., MUNKNER T. Transferrin in normal cerebrospinal fluid. Nature. 1961 Jan 7;189:60–61. doi: 10.1038/189060b0. [DOI] [PubMed] [Google Scholar]
- Fletcher J., Huehns E. R. Function of transferrin. Nature. 1968 Jun 29;218(5148):1211–1214. doi: 10.1038/2181211a0. [DOI] [PubMed] [Google Scholar]
- Greene F. C., Feeney R. E. Physical evidence for transferrins as single polypeptide chains. Biochemistry. 1968 Apr;7(4):1366–1371. doi: 10.1021/bi00844a018. [DOI] [PubMed] [Google Scholar]
- Jeppsson J. O. Isolation and partial characterization of three human transferrin variants. Biochim Biophys Acta. 1967 Aug 15;140(3):468–476. doi: 10.1016/0005-2795(67)90519-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Løwenstein H. Quantitative immunoelectrophoretic methods as a tool for the analysis and isolation of allergens. Prog Allergy. 1978;25:1–62. doi: 10.1159/000314432. [DOI] [PubMed] [Google Scholar]
- Mann K. G., Fish W. W., Cox A. C., Tanford C. Single-chain nature of human serum transferrin. Biochemistry. 1970 Mar 17;9(6):1348–1354. doi: 10.1021/bi00808a008. [DOI] [PubMed] [Google Scholar]
- PARKER W. C., BEARN A. G. Studies on the transferrins of adult serum, cord serum, and cerebrospinal fluid. The effect of neuraminidase. J Exp Med. 1962 Jan 1;115:83–105. doi: 10.1084/jem.115.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho-Pinto F. J., McLaren D. J., Smithers S. R. Complement-mediated killing of schistosomula of Schistosoma mansoni by rat eosinophils in vitro. J Exp Med. 1978 Jan 1;147(1):147–156. doi: 10.1084/jem.147.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner M. Identification of the binding site for transferrin in human reticulocytes. Biochem Biophys Res Commun. 1980 Jun 16;94(3):861–866. doi: 10.1016/0006-291x(80)91314-5. [DOI] [PubMed] [Google Scholar]
- Sutherland R., Delia D., Schneider C., Newman R., Kemshead J., Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H. G., Hass P. E., Sussman H. H. Transferrin receptor in human placental brush border membranes. Studies on the binding of transferrin to placental membrane vesicles and the identification of a placental brush border glycoprotein with high affinity for transferrin. J Biol Chem. 1979 Dec 25;254(24):12629–12635. [PubMed] [Google Scholar]