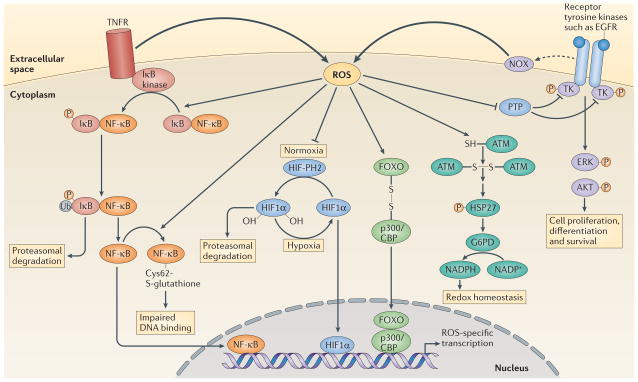
Figure 3. Examples of transcriptional regulation by ROS acting at the plasma membrane or in the cytosol.
Activation of tumour necrosis factor receptor (TNFR) triggers reactive oxygen species (ROS) production, which enhances the phosphorylation (P) of inhibitor of NF-κB (IκB), probably through the oxidative inhibition of a phosphatase. This leads to the ubiquitylation (Ub) of IκB and its subsequent degradation by the proteasome. Nuclear factor-κB (NF-κB) is then released and translocates to the nucleus to initiate transcription. ROS production can also trigger oxidative glutathionylation of NF-κB at its redox sensitive cysteine, which reduces its DNA binding affinity133. Under normoxia, prolyl hydroxylases (PHs) hydroxylate hypoxia-inducible factor 1α (HIF11α), which allows it to be recognized by the E3 ligase von Hippel–Lindau tumour-suppressor protein (VHL) and promotes its degradation by the proteasome. Under hypoxia there is increased production of ROS (FIG. 4), which inhibits prolyl hydroxylases, leading to the accumulation of HIF11α. HIF11α then translocates to the nucleus to mediate gene transcription. ROS production by NADPH oxidases (NOXs) following receptor activation by specific ligands, for example, epidermal growth factor receptor (EGFR), inhibits protein tyrosine phosphatases (PTPs), which promotes the phosphorylation of tyrosine kinases (TKs) and the subsequent signal transduction. By contrast, ataxia-telangiectasia mutated (ATM) kinase is activated directly by ROS, through disulphide bond-mediated homodimerization, which leads to the phosphorylation of heat shock protein 27 (HSP27) and the subsequent activation of glucose-6-phosphate-dehydrogenase (G6PD). The resulting increase in NADPH levels contributes to the maintenance of cellular redox homeostasis. ROS-mediated disulphide bonding can also lead to heterodimerization, as seen between forkhead box O (FOXO) transcription factors and p300/CBP acetyltransferase, which leads to the acetylation of FOXO proteins and the activation of specific gene transcription. ERK, extracellular signal-regulated kinase.