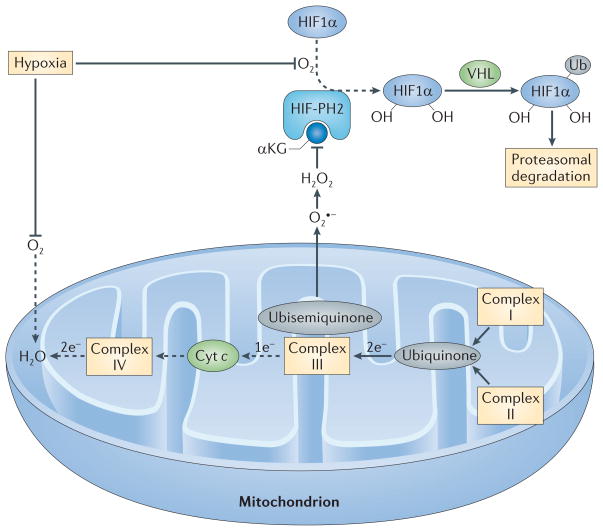
Figure 4. Regulation of HIF1α by mitochondrial ROS production during hypoxia.
In the presence of O2 and its cofactor α-ketoglutarate (αKG), HIF-prolyl hydroxylase 2 (HIF-PH2) hydroxylates two proline residues in hypoxia-inducible factor 1α (HIF1α). Hydroxylated HIF1α is then ubiquitylated (Ub) by the E3 ligase von Hippel–Lindau tumour-suppressor protein (VHL) and is subsequently degraded by the proteasome. Because O2 is a substrate for HIF-PH2, hypoxia limits HIF-PH2 activity. This limitation is enhanced by the negative effect of hypoxia-driven mitochondrial reactive oxygen species (ROS) on HIF-PH2 function. Complex III in the mitochondrial electron transport chain (METC) receives two electrons from ubiquinone but can only transfer one electron at a time to cytochrome c (Cyt c). Complex III therefore transfers one electron to a quasi-stable ubisemiquinone radical. If this radical accumulates, O2 that is dissolved in the mitochondrial membrane can capture the electron before cytochrome c accepts it, generating superoxide (O2•−). If cellular O2 is low, complex IV at the end of the METC is slow to transfer pairs of electrons to O2 (making water), and the METC is blocked between complex III and IV, which favours the electron leak described above. The newly formed superoxide dismutates to H2O2. H2O2 can inhibit HIF-PH2 and oxidatively decarboxylate its cofactor, αKG. The resulting decrease in the hydroxylation of HIF1α and its subsequent proteasomal degradation supports HIF1α accumulation. Dashed arrows indicate the reactions that are slowed in hypoxia.