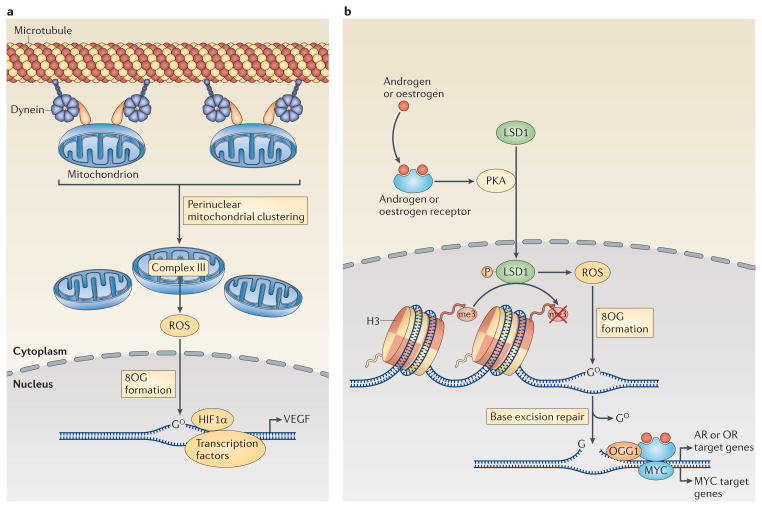
Figure 5. Regulation of transcription through DNA targeting by intranuclear ROS.
a | The induction of the transcription of the gene encoding vascular endothelial growth factor (VEGF) by hypoxia-inducible factor 1α (HIF1α) is enhanced through the dynein-mediated, perinuclear localization of mitochondria. Mitochondria-derived reactive oxygen species (ROS) diffuse into the nucleus, where they promote the oxidation of guanine nucleotides, forming 8-oxoguanine (8OG). b | Transcriptional regulation downstream of the activation of androgen receptors (ARs) or oestrogen receptors (ORs) and other nuclear receptors also involves DNA modifications by ROS. Engagement of ORs or ARs promotes the phosphorylation (P) of lysine-specific histone demethylase 1A (LSD1) by cAMP-dependent protein kinase (PKA). Active LSD1 not only demethylates histone 3 (H3) but also produces ROS, which then promote the formation of 8OG in the DNA. The altered DNA bases recruit base excision repair machinery, and the DNA breaks that are generated by 8OG DNA glycosylase 1 (OGG1) enable the activation of transcription by AR, OR and possibly also MYC.