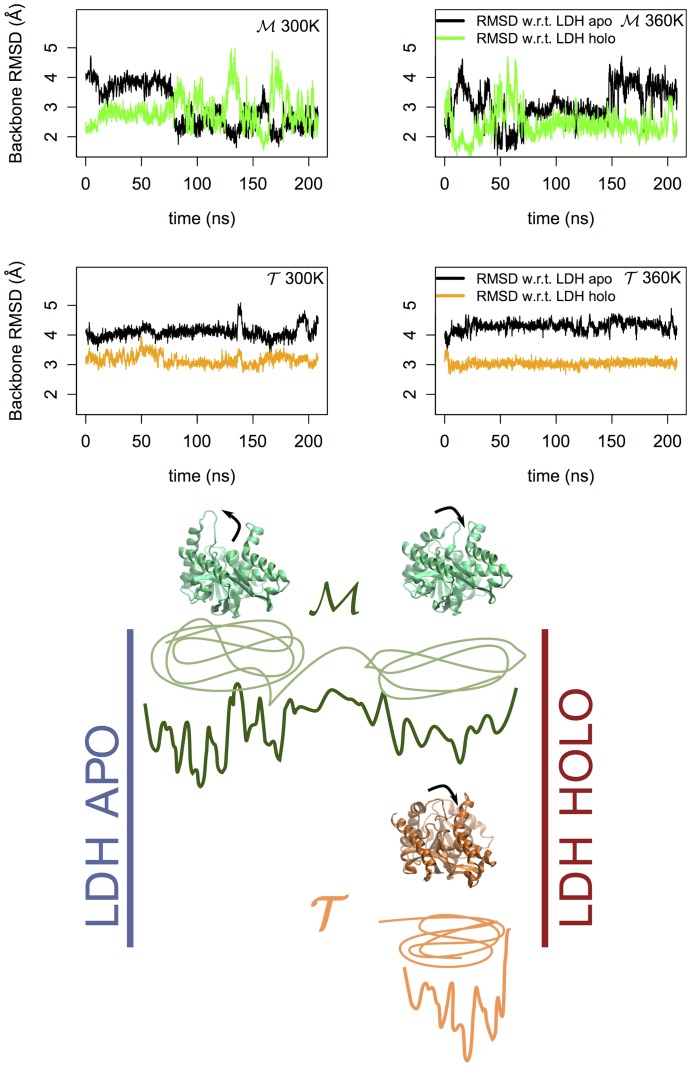
Figure 7. Catalytic pocket dynamics.
In the upper part of the figure we report the RMSD computed using the backbone heavy atoms of the residues that form the catalytic pocket of 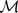 (upper graphs) and
(upper graphs) and 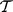 (bottom graphs) w.r.t. those of the apo and holo forms of lactate dehydrogenase at 300 K (left) and 360 K (right). In the lower part of the figure we present a pictorial representation of the conformational states accessible by the proteins when considered as function of the distance with respect to LDH apo and holo conformers. In green we sketched the two states visited by the
(bottom graphs) w.r.t. those of the apo and holo forms of lactate dehydrogenase at 300 K (left) and 360 K (right). In the lower part of the figure we present a pictorial representation of the conformational states accessible by the proteins when considered as function of the distance with respect to LDH apo and holo conformers. In green we sketched the two states visited by the 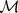 MalDH, one characterized by an open conformation of the binding site loop and the other associated to a closed state. The
MalDH, one characterized by an open conformation of the binding site loop and the other associated to a closed state. The  MalDH (orange) is instead tightly confined in a closed state.
MalDH (orange) is instead tightly confined in a closed state.