Abstract
AIM: To study the core cell damage in isolated islets of Langerhans and its prevention by low temperature preconditioning (26 °C).
METHODS: Islets were cultured at 37 °C for 7-14 d after isolation, and then at 26 °C for 2, 4 and 7 d before additional culture at 37 °C for another 7 d. Core cell damage in the isolated islets was monitored by video-microscopy and analyzed quantitatively by use of a computer-assisted image analysis system. The analysis included daily measurement of the diameter and the area of the isolated islets and the area of the core cell damage that developed in those islets over time during culture. Histology and TdT-mediated dUTP-biotin nick end labeling (TUNEL) assay were used to characterize the cell damage and to monitor islet function.
RESULTS: Microscopic analysis showed that during the 7 to 14 d of culture at 37 °C, core cell damage occurred in the larger islets with diameters >200 μm, which included both necrotic and apoptotic cell death. Low temperature (26 °C) culture could prevent core cell damage of isolated islets. The 7-d culture procedure at 26 °C could inhibit most of the core cell (excluding diameters>300 μm) damages when the islets were re-warmed at 37 °C.
CONCLUSION: Our results indicate that core cell damage within isolated islets of Langerhans correlates with the size of islets. Low temperature (26 °C) culture can prevent core cell damage in isolated islets, and successfully precondition these islets for incubation at 37 °C. These novel findings may help to understand the pathophysiology of early loss of islet tissue after transplantation, and may provide a new strategy to improve graft function in the clinical setting of islet transplantation.
Keywords: Islets of Langerhans, Low temperature preconditioning, Core cell damage
INTRODUCTION
At present, a considerable number of studies have demonstrated that the reasons for the failure of islet transplantation (besides graft rejection) are the loss of islet number and function[1-8]. Our previous studies demonstrate that freely transplanted graft islets are considered avascular as early as 2 d after transplantation[9], and hypoxia might induce islet cell damage (necrosis and apoptosis). In fact, necrosis occurs in islets maintained in vitro[10-15]. Core islets are primarily constituted by β-cells[16,17], which is in line with the fact that most affected (apoptotic) cells after isolation are beta-cells[18]. The prolonged survival of allogenetically transplanted islets is correlated with low temperature, which, however, is interpreted as a low temperature-associated immunomodulatory function[10,19-21]. And the view that well-preserved islets or β-cells are procured by low temperature has been elucidated in in vitro experiments[22-25] . In this experimental study, we investigated whether low temperature preconditioning (26 °C) could prevent core cell damage in isolated islets before transplantation.
MATERIALS AND METHODS
Animals
Inbred Syrian golden hamsters weighing 127-297 g were used. Animals were housed per cage at the beginning of each experiment and allowed free access to tap water and standard pellet food.
Islet isolation
A modified method by stationary digestion after pancreatic ductal injection of collagenase solution was used[26]. Briefly, hamsters were anesthetized with pentobarbital by intraperitoneal injection at 50 mg/kg body weight. For the exposure of the whole pancreas, the abdominal wall was opened via a midline incision. With the help of a stereo-microscope (Wild, Heerbrugg, Switzerland), the common bile duct was first ligated at its entrance to the liver in order to prevent collagenase solution retrograde entering the liver, and then the pancreatic duct proximal to the duodenum was exposed. Then cannulation of the pancreatic duct was performed using a polyethylene catheter, and connected to a syringe filled with 5 mL of Dulbecco’s phosphate-buffered saline solution. This solution additionally contained 8 mg collagenase V (Sigma, Deisenhofen, Germany) per 10 mL PBS without Ca++ and Mg++, and 0.1 mg/mL neutral red (Sigma) for islet staining. The aorta was transected immediately to minimize interstitial hemorrhage in the pancreas before the duct was retrograde injected with PBS solution. Red colour on the surface of pancreas gradually appeared and the pancreas began to be distended during that time. Finally, the whole pancreas was excised and put into a Petri dish containing another 5 mL of PBS solution. The Petri dish with the pancreatic tissue in the PBS solution was immediately placed in an incubator at 37 °C in 950 mL/L O2/50 mL/L CO2 for 20-25 min to further allow tissue digestion. Then, the Petri dish was taken out and put on ice to stop the digestion process. Tissue suspensions were then washed two to three times at 10 min intervals, and passed through a filter net (800 μm pores) in 5 mL of PBS solution. Finally, the islets were handpicked with a pipette under a stereomicroscope and transferred to two Petri dishes. One Petri dish was incubated at 37 °C in a humidified atmosphere containing 950 mL/L O2/50 mL/L CO2 for 7-14 d as control. Another was incubated at low temperature (26 °C, 950 mL/L O2/50 mL/L CO2). Each Petri dish contained 450 mL of Dulbecco’s modified Eagle’s medium (DMEM, PAA Laboratories GmbH, Linz, Austria), 50 mL of fetal calf serum (FCS) and 50 mg of gentamycin. The number of freshly isolated islets from one hamster usually was about 200-300.
Low temperature culture (26 °C)
Petri dishes for preconditioning and control were incubated at 26 °C for 7 d. These experiments included ten culture procedures in 10 donor animals, each culture procedure containing about 50 to 80 islets.
Re-warming after low temperature
Freshly isolated islets in the culture medium were incubated at 26 °C for 2, 4 and 7 d respectively, and then re-warmed 37 °C for a further 7 d. Controls were incubated at 37 °C for 7 d. After culturing at 26 °C for 2,4 and 7 d, these experiments included 4, 3 and 5 culture procedures in 4, 3 and 5 donor animals, respectively and each culture procedure contained about 50 to 80 islets.
Microscopic monitoring of cultured islets
Morphological changes of isolated islets were monitored during 7 to 14 d of culture. Images were recorded by means of optronics engineering device (TEC-470; SI GmbH, Gilching, Germany), and finally transferred to a Super VHS ET video recorder (JVC, Germany) for off-line evaluation. To evaluate the core cell damage of isolated islets, microscopic analyses were performed at different time points ( 0-, 1-, 2-, 3-, 4-, 5-, 7-, and/or 14-d) during islet culture.
Analysis of cell death by computer-assisted measures
The diameter and area of the islets as well as the area of the core cell damage were quantified by using a computer-assisted image analysis system (CapImage, Zeintl, Heidelberg, Germany). The diameter of the islets was measured in two modes, horizontal and vertical, and presented as mean value. The areas of the islets and the core cell damage were analyzed planimetrically. In case several intraislet areas of cell damage were present, those areas were measured individually, then added to the overall damaged area. Finally, the data on the area of cell damage were expressed as percent of the area of the islet.
Histology and apoptosis within the core cell damage in isolated islets
Some islets were harvested as specimens at different time points, fixed in 40 mL/L phosphate-buffered formalin for 2-3 d, embedded in paraffin and sectioned for staining with hematoxylin-eosin (H-E) for routine histology. To identify whether apoptosis was involved in core cell damage in isolated islets and which cells in the islets underwent apoptosis, the TUNEL assay was employed. Briefly, islets with core cell damage were selected and fixed in 40 g/L paraformaldehyde, sectioned in paraffin and pretreated with 1 g/L trypsin buffer at 37 °C for 5 min. The in situ cell death detection kit (Boehringer Mannheim) was used for the labeling of apoptotic cells. TUNEL-positive cells containing damaged DNA were marked by an anti-fluorescein antibody conjugated with horseradish peroxidase and developed a dark brown color in the nuclei.
Statistical analysis
Data were presented as mean±SE. After one way analysis of variance (ANOVA), comparison between groups was tested by the appropriate post-hoc test. Differences were considered statistically significant at P<0.05. Statistics were calculated using the software package SigmaStat (Jandel Corporation, San Rafael, CA).
RESULTS
Core cell damage of isolated islets
Under light microscope, freshly isolated pancreatic islets of Langerhans from Syrian golden hamsters had a smooth appearance with compact, differently spherical shapes, varied in size (Figure 1). After 1 d culture at 37 °C, the islets began to present some cell damage. Usually, this was found to be located in the center of the islet and was characterized by a zone of very dark cells that was sharply separated from the surrounding viable islet tissue. By d 2 of culture, an extensive area of damaged cells in the central islet region was apparent (Figure 2).
Figure 1.
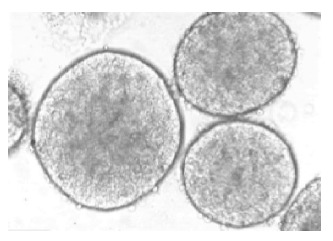
Freshly isolated islets of Langerhans from Syrian golden hamsters. The smooth appearance of the islets’ shape, and the variation in size could be observed. Magnification ×120.
Figure 2.
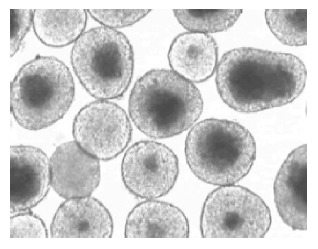
Core cell damage of isolated islets after 2-d culture at 37 °C in 950 mL/L O2/50 mL/L CO2 atmosphere. The core cell damage occurred predominantly in the larger islets. Magnification ×70.
By routine histology staining with H-E, the centrally altered area was shown to be composed of damaged β-cells in the islet core (Figure 3). With TUNEL assay, apoptotic cell death of single damaged cells within the core of the 2-d cultured islets could be identified. However, many cells within this focus were also TUNEL negative, indicating that both necrotic and apoptotic cell deaths were involved in the process of core cell damage in 37 °C cultured isolated pancreatic islets (Figure 4).
Figure 3.
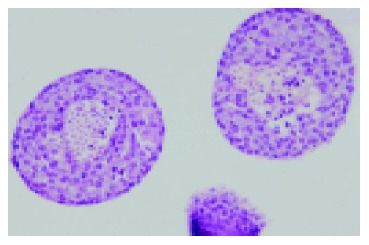
HE staining of isolated islets after 2-d culture at 37 °C in 950 mL/L O2/50 mL/L CO2 atmosphere (Magnification ×100). The core β-cell damage occurred within these larger islets.
Figure 4.
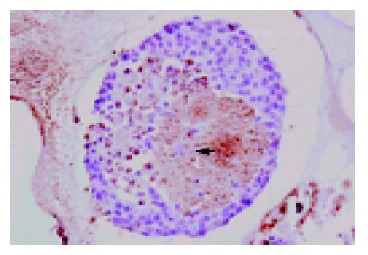
TUNEL assay of isolated islets after 2-d culture at 37 °C in 950 mL/L O2/50 mL/L CO2 atmosphere (Magnification ×120). TUNEL-positive cells containing damaged DNA developed a dark brown color in the nuclei, indicating cell apoptosis.
In order to make a detailed analysis, islets were grouped into 4 categories according to their individual diameters: group A, islets with diameters <100 μm; group B, islets with diameters between 100 μm and 200 μm; group C, islets with diameters between 200 μm and 300 μm; and group D, islets with diameters >300 μm. After calculating the percent of core cell damage of each group during the 7-d culture at 37 °C, we found that in group C, and particularly in group D, islets showed significant core cell damage while the small islets of groups A and B did not reveal any core cell damage. This result was taken as a control for low temperature precondition.
Effect of low temperature on core cell damage in isolated islets
In this experiment, the culture of isolated islets was incubated under low temperature (26 °C). Strikingly, low temperature culture did not lead to core cell damage during the 7-d culture. Intact histological structure of these islets after the 7-d culture period at 26 °C was observed (Figure 5).
Figure 5.
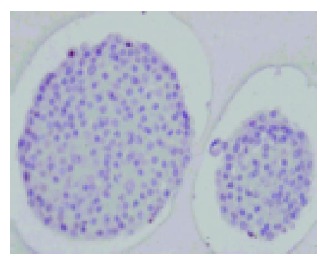
Intact histological structure of isolated islets after a 7-d culture at 26 °C in 950 mL/L O2/50 mL/L CO2 atmosphere (H-E staining). Magnification ×150.
Because consecutive 7-d re-warming culture experiments at low temperature after 2, 4, or 7 d extended the overall 7-d observation period, we additionally performed control culture experiments of isolated islets at 37 °C for 14 d. These experiments demonstrated that the degree of core cell damage had no difference in 14-d cultured islets when compared with those underwent only a 7-d culture period, indicating that the magnitude of core cell damage might be determined within the first 7 d of cell culture (hypoxia). Islets after 2-d and 4-d culture at 26 °C were re-warmed at 37 °C, and incubation was continued at 37 °C for another 7 d. These experiments revealed that the 2-d and 4-d low temperature cultures were not effective to prevent core cell damage of islets with diameters >200 μm, indicating that the 2-d and 4-d low temperature conditioning was not successful to protect the isolated islets from hypoxia-induced injury under warm conditions (Figures 6, 7).
Figure 6.
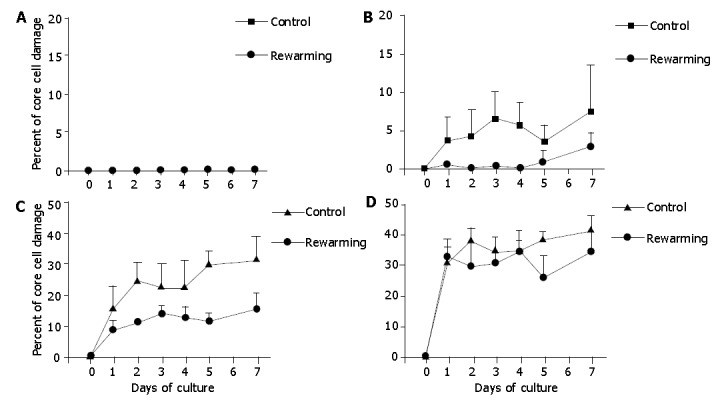
Core cell damage in re-warmed islets with diameters <100 μm (A), 100-200 μm (B), 200-300 μm (C), and >300 μm (D) after a 2-d culture preconditioning at 26 °C during a 7-d culture at 37 °C. Islets cultured only at 37 °C served as controls. The 2-d culture preconditioning at 26 °C did not prevent core cell damage in islets with diamerer >100 μm under 37 °C culture conditions.
Figure 7.
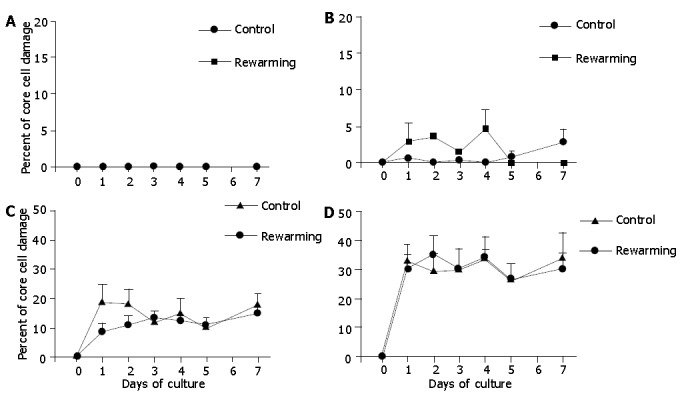
Core cell damage in rewarmed islets with diameters <100 μm (A), 100-200 μm (B), 200-300 μm (C), and >300 μm (D) after a 4-d culture at 26 °C during a 7-d culture at 37 °C. Islets cultured only at 37 °C served as controls. The 4-d culture preconditioning at 26 °C did not prevent core cell damage of islets with diameter>100 μm under 37 °C culture conditions.
Islets after a 7-d culture at 26 °C were re-warmed at 37 °C, and incubation was continued at 37 °C for another 7 d. These experiments revealed that a 7-d low temperature culture was indeed effective to reduce core cell damage of islets with diameters >100 μm, indicating that the 7-d low temperature conditioning might be a promising tool to protect the isolated islets from hypoxia-induced injury under warm conditions (Figure 8).
Figure 8.
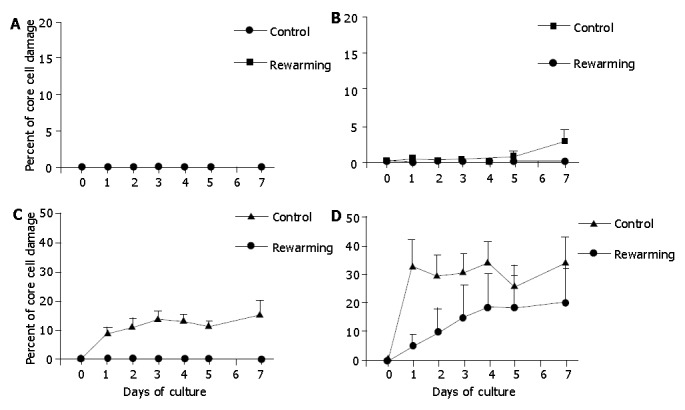
Re-warmed islets with diameters <100 μm (A), 100-200 μm (B), 200-300 μm (C), and >300 μm (D) after a 7-d culture at 26 °C during an additional 7-d culture at 37 °C. Islets cultured only at 37 °C served as controls. The 7-d culture preconditioning at 26 °C was effective to reduce core cell damage of islets with diameter >100 μm under 37 °C culture conditions. aP<0.05 vs Controls.
DISCUSSION
The major obstacles for successful clinical islet transplantation are the isolation of a sufficient mass of islets and the management of graft rejection[27,28] . Although recent achievements in attaining a high number of islets and a renewed immunosuppression protocol are introduced with a somewhat more promising outcome[29-31], the problem of early loss of function after transplantation has not been solved yet. This fatal outcome, which is not related to immune rejection, has been inferentially thought to be due to insufficient or non-established vascularization of transplanted islets[32-34]. In our previous studies[10-13], we demonstrated that freely transplanted islets showed the first signs of angiogenesis (i.e., capillary sprout formation and protrusion) as early as 2 d after transplantation, and the entire vascularization process was completed after 10 to 14 d[9]. Thus, for the first two days after transplantation, the grafted islets have to be considered avascular, and the nutritional supply is provided only by diffusion from host blood vessels. This may be sufficient for the peripheral β-cells. However, the central area of the islet grafts, which is composed predominantly of β-cells, will not receive an adequate nutritional supply. In fact, several studies have pointed out the development of central necrosis in isolated pancreatic islets[11]. This experiment demonstrated a correlation between core cell damage in isolated islets of Langerhans and the size of islets (diameter and area). We found that the islets with diameters >200 μm were more sensitive to core cell damage than islets with diameters <200 μm. In the islets with diameters <100 μm, no core cell damage occurred at all during the 7-d culture period at 37 °C. This finding is in line with the currently discussed minimal requirement for islet size, which measures 150 μm in diameter[35].
There might be two causative mechanisms involved in the core cell damage in the isolated islets. First, necrotic cell death, as mentioned above, is generally believed to be due to limitation of nutrition-diffusion. This is demonstrated in part by the observation that usually it is the center of the larger islets that are mainly susceptible to lack of nutrients, which finally results in cell necrosis[11]. It is indeed the case when islets are cultured in medium environment, which is similar to that of host blood after transplantation. Second, the isolation procedure of islets generally destroys the islet microenvironment and places islets in an atmosphere of pathological stimuli, including loss of trophic support, alteration of osmosis and entrancement of some endotoxins in collagenase[18]. Moreover, islets are notorious for their high sensitivity to noxious stimuli. Hence, the occurrence of apoptosis in freshly isolated islets may be termed isolation-induced islet cell apoptosis. This view has been confirmed in the present study by TUNEL assay analysis. We actually do not know which type of cell death is the more dominant in large islet core cell damage. Perhaps two kinds of mechanisms co-exist.
Core cell damage in larger islets occurred during the 7-d culture at 37 °C, but at 26 °C, no damage occurred. Therefore, pre-incubation at low temperature could help prevent core cell damage, and a 7-d culture at 26 °C could completely prevent core cell damage during a subsequent 7-d culture at 37 °C, when the islets maintain a well-preserved morphologic structure. It is possible that lower temperature at 26 °C decreases islet cell metabolic activity and oxygen consumption, especially in β-cells, and increases the islet cellular ability to tolerate hypoxia, thereby avoiding core cell necrosis and apoptosis. Furthermore, the oxygen solubility in liquids increases as the temperature is reduced, which leads to oxygen concentrations in the culture medium, and the oxygen diffusion distance to islet cores may be shortened[19].
In the present study, the functional parameters of cultured islets at 26 °C, such as insulin content and secretion, were not studied. However, previous studies on rat islets clearly show that the ability of glucose to stimulate insulin release at low temperature remains stable for three weeks, and increasing the temperature to 37 °C after one-week culture produced a 7-fold increase in the rate of insulin release[10].
In view that re-warming may recover insulin release of cultured islets at low temperature, we question whether re-warming of the islets at 37 °C after culture for several days at 26 °C could also prevent core cell damage. However, both 2-d and 4-d low temperature (26 °C) culture conditionings were not effective to prevent the development of core cell damage in islets with diameter >200 μm under 37 °C warm conditions. In contrast, prolonging 26 °C preconditioning to 7 d resulted in a marked reduction of core cell damage after re-warming of the islets at 37 °C. Only very large islets showed some central alterations. However, even though those largest islets showed core cell damage, the extent of damage was not as serious as observed in non-preconditioned islets.
In conclusion, core cell damage within isolated islets of Langerhans correlates with the size of islets. Low temperature (26 °C) culture can diminish core cell damage in isolated islets. Also, a 7-d culture procedure at 26 °C can inhibit most core cell damages when the islets are re-warmed at 37 °C. These novel findings may encourage in vivo studies on islet transplantation after preconditioning the isolated islets at low temperature (26 °C). In fact, protection from core cell damage in transplanted islets may markedly improve the final outcome by reducing the high frequency of non-functional primary grafts.
Footnotes
Supported by the National Natural Science Foundation of China, No. 30271274 and German DAAD-Wong Kuan Cheng Fellowship
Edited by Kumar M and Wang XL Proofread by Zhu LH
References
- 1.Federlin KF. Islet transplantation. The connection of experiment and clinic exemplified by the transplantation of islets of Langerhans. Exp Clin Endocrinol. 1993;101:334–345. doi: 10.1055/s-0029-1211254. [DOI] [PubMed] [Google Scholar]
- 2.Kendall DM, Robertson RP. Pancreas and islet transplantation. Challenges for the twenty-first century. Endocrinol Metab Clin North Am. 1997;26:611–630. doi: 10.1016/s0889-8529(05)70270-x. [DOI] [PubMed] [Google Scholar]
- 3.Kenyon NS, Alejandro R, Mintz DH, Ricordi C. Islet cell transplantation: beyond the paradigms. Diabetes Metab Rev. 1996;12:361–372. doi: 10.1002/(SICI)1099-0895(199612)12:4<361::AID-DMR172>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 4.Brunicardi FC, Mullen Y. Issues in clinical islet transplantation. Pancreas. 1994;9:281–290. doi: 10.1097/00006676-199405000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Menger MD, Messmer K. Pancreatic islet transplantation: isolation, separation, and microvascularization. Wien Klin Wochenschr. 1992;104:429–433. [PubMed] [Google Scholar]
- 6.Menger MD, Pattenier J, Wolf B, Jäger S, Feifel G, Messmer K. Cryopreservation of islets of Langerhans does not affect angiogenesis and revascularization after free transplantation. Eur Surg Res. 1992;24:89–96. doi: 10.1159/000129193. [DOI] [PubMed] [Google Scholar]
- 7.Vajkoczy P, Menger MD, Simpson E, Messmer K. Angiogenesis and vascularization of murine pancreatic islet isografts. Transplantation. 1995;60:123–127. [PubMed] [Google Scholar]
- 8.Beger C, Cirulli V, Vajkoczy P, Halban PA, Menger MD. Vascularization of purified pancreatic islet-like cell aggregates (pseudoislets) after syngeneic transplantation. Diabetes. 1998;47:559–565. doi: 10.2337/diabetes.47.4.559. [DOI] [PubMed] [Google Scholar]
- 9.Menger MD, Jaeger S, Walter P, Feifel G, Hammersen F, Messmer K. Angiogenesis and hemodynamics of microvasculature of transplanted islets of Langerhans. Diabetes. 1989;38 Suppl 1:199–201. doi: 10.2337/diab.38.1.s199. [DOI] [PubMed] [Google Scholar]
- 10.Ono J, Lacy PE, Michael HE, Greider MH. Studies of the functional and morphologic status of islets maintained at 24 C for four weeks in vitro. Am J Pathol. 1979;97:489–503. [PMC free article] [PubMed] [Google Scholar]
- 11.Ilieva A, Yuan S, Wang RN, Agapitos D, Hill DJ, Rosenberg L. Pancreatic islet cell survival following islet isolation: the role of cellular interactions in the pancreas. J Endocrinol. 1999;161:357–364. doi: 10.1677/joe.0.1610357. [DOI] [PubMed] [Google Scholar]
- 12.de Graaff MP, Wolters GH, van Schilfgaarde R. Endothelial cells in pancreatic islets and the effect of culture. Transplant Proc. 1994;26:1171. [PubMed] [Google Scholar]
- 13.Metrakos P, Yuan S, Qi SJ, Duguid WP, Rosenberg L. Collagen gel matrix promotes islet cell proliferation. Transplant Proc. 1994;26:3349–3350. [PubMed] [Google Scholar]
- 14.Hart TK, Pino RM. Pseudoislet vascularization. Induction of diaphragm-fenestrated endothelia from the hepatic sinusoids. Lab Invest. 1986;54:304–313. [PubMed] [Google Scholar]
- 15.Vajkoczy P, Olofsson AM, Lehr HA, Leiderer R, Hammersen F, Arfors KE, Menger MD. Histogenesis and ultrastructure of pancreatic islet graft microvasculature. Evidence for graft revascularization by endothelial cells of host origin. Am J Pathol. 1995;146:1397–1405. [PMC free article] [PubMed] [Google Scholar]
- 16.Maglio M, Putti R. Morphological basis of the interactions between endocrine cell types in the pancreatic islets of the teleost, Blennius gattoruggine. Tissue Cell. 1998;30:672–683. doi: 10.1016/s0040-8166(98)80086-4. [DOI] [PubMed] [Google Scholar]
- 17.Putti R, Della Rossa A, Maglio M, Tagliafierro G. Islets and diffuse endocrine component in the pancreas of three red frogs species: relationships between endocrine and exocrine tissue. Tissue Cell. 1997;29:355–363. doi: 10.1016/s0040-8166(97)80011-0. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg L, Wang R, Paraskevas S, Maysinger D. Structural and functional changes resulting from islet isolation lead to islet cell death. Surgery. 1999;126:393–398. [PubMed] [Google Scholar]
- 19.Brandhorst D, Brandhorst H, Hering BJ, Bretzel RG. Long-term survival, morphology and in vitro function of isolated pig islets under different culture conditions. Transplantation. 1999;67:1533–1541. doi: 10.1097/00007890-199906270-00006. [DOI] [PubMed] [Google Scholar]
- 20.Lacy PE, Davie JM, Finke EH. Prolongation of islet xenograft survival without continuous immunosuppression. Science. 1980;209:283–285. doi: 10.1126/science.6770465. [DOI] [PubMed] [Google Scholar]
- 21.Bobzien B, Yasunami Y, Majercik M, Lacy PE, Davie JM. Intratesticular transplants of islet xenografts (rat to mouse) Diabetes. 1983;32:213–216. doi: 10.2337/diab.32.3.213. [DOI] [PubMed] [Google Scholar]
- 22.Frankel BJ, Gylfe E, Hellman B, Idahl LA. Maintenance of insulin release from pancreatic islets stored in the cold for up to 5 weeks. J Clin Invest. 1976;57:47–52. doi: 10.1172/JCI108267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tai J, Tsang A, Tze WJ. Effects of serum and medium supplements, pH, and temperature on the viability of cultured porcine islets. Transplant Proc. 1994;26:818–819. [PubMed] [Google Scholar]
- 24.Korbutt GS, Pipeleers DG. Cold-preservation of pancreatic beta cells. Cell Transplant. 1994;3:291–297. doi: 10.1177/096368979400300405. [DOI] [PubMed] [Google Scholar]
- 25.Menger MD, Jäger S, Walter P, Hammersen F, Messmer K. A novel technique for studies on the microvasculature of transplanted islets of Langerhans in vivo. Int J Microcirc Clin Exp. 1990;9:103–117. [PubMed] [Google Scholar]
- 26.Gotoh M, Maki T, Satomi S, Porter J, Bonner-Weir S, O'Hara CJ, Monaco AP. Reproducible high yield of rat islets by stationary in vitro digestion following pancreatic ductal or portal venous collagenase injection. Transplantation. 1987;43:725–730. doi: 10.1097/00007890-198705000-00024. [DOI] [PubMed] [Google Scholar]
- 27.Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992;72:1063–1081. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- 28.Groth CG, Hering BJ, Geier C, Bretzel RG, Federlin K. Immunosuppression in pancreatic islet cell transplantation. Transplant Proc. 1994;26:2756. [PubMed] [Google Scholar]
- 29.Ryan EA, Lakey JR, Rajotte RV, Korbutt GS, Kin T, Imes S, Rabinovitch A, Elliott JF, Bigam D, Kneteman NM, et al. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes. 2001;50:710–719. doi: 10.2337/diabetes.50.4.710. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro AM, Gallant H, Hao E, Wong J, Rajotte R, Yatscoff R, Kneteman N. Portal vein immunosuppressant levels and islet graft toxicity. Transplant Proc. 1998;30:641. doi: 10.1016/s0041-1345(97)01441-3. [DOI] [PubMed] [Google Scholar]
- 31.Gremlich S, Roduit R, Thorens B. Dexamethasone induces posttranslational degradation of GLUT2 and inhibition of insulin secretion in isolated pancreatic beta cells. Comparison with the effects of fatty acids. J Biol Chem. 1997;272:3216–3222. doi: 10.1074/jbc.272.6.3216. [DOI] [PubMed] [Google Scholar]
- 32.Berney T, Ricordi C. Islet cell transplantation: the future? Langenbecks Arch Surg. 2000;385:373–378. doi: 10.1007/s004230000118. [DOI] [PubMed] [Google Scholar]
- 33.Sun Y, Ma X, Zhou D, Vacek I, Sun AM. Normalization of diabetes in spontaneously diabetic cynomologus monkeys by xenografts of microencapsulated porcine islets without immunosuppression. J Clin Invest. 1996;98:1417–1422. doi: 10.1172/JCI118929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Behboo R, Carroll PB, Trucco M, Ricordi C. Decreased nitric oxide generation following human islet culture in serum-free media. Transplant Proc. 1995;27:3380. [PubMed] [Google Scholar]
- 35.Bretzel RG. Current status and perspectives in clinical islet transplantation. J Hepatobiliary Pancreat Surg. 2000;7:370–373. doi: 10.1007/s005340070031. [DOI] [PubMed] [Google Scholar]


