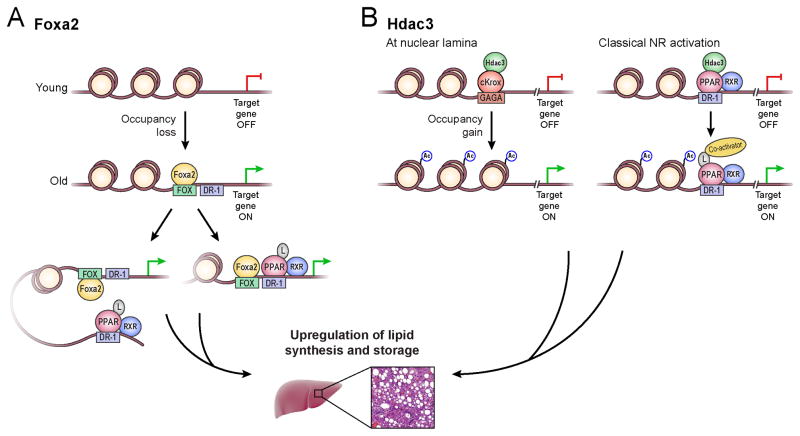
Figure 6. A model relating chromatin changes to development of fatty liver during aging.
Change in nucleosome occupancy in aged liver is associated with upregulation of nuclear receptor targets and development of steatosis. (A) Foxa2 binding leads to nucleosome eviction in older livers. Foxa2 cooperates with ligand-activated PPAR receptors (L: ligand), either interacting with existing PPAR proteins bound to enhancer elements (left) or enabling additional PPAR binding at the promoter (right), leading to upregulation of targets regulating lipid synthesis and storage. (B) Hdac3 regulates hepatic lipid targets in two ways: 1) at the nuclear lamina through GAGA sites bound by cKrox/Hdac3 (left), and 2) by repressing PPAR sites in young but not old livers in a classical mechanism of nuclear receptor (NR) action. Regions with a GAGA motif, bound by cKrox (Zbtb7b) in complex with Hdac3, inhibit expression of lipogenic targets in young livers. Age-dependent gain in nucleosome occupancy at these locations leads to eviction of histone deacytelase Hdac3. Nucleosomes can now be acetylated, leading to active transcription of nuclear receptor targets. In addition, in a classical model of nuclear receptor activation, Hdac3 binds unliganded PPARα in young livers and is evicted upon agonist stimulation in old livers. A co-activator with histone acetyltransferase activity is recruited to the PPAR complex. Nearby nucleosomes are acetylated and gene expression of the targets is turned on. The reciprocal binding pattern of Foxa2 and Hdac3 at loci encoding PPARα targets contributes to dysregulation of hepatic lipid homeostasis during aging.