Abstract
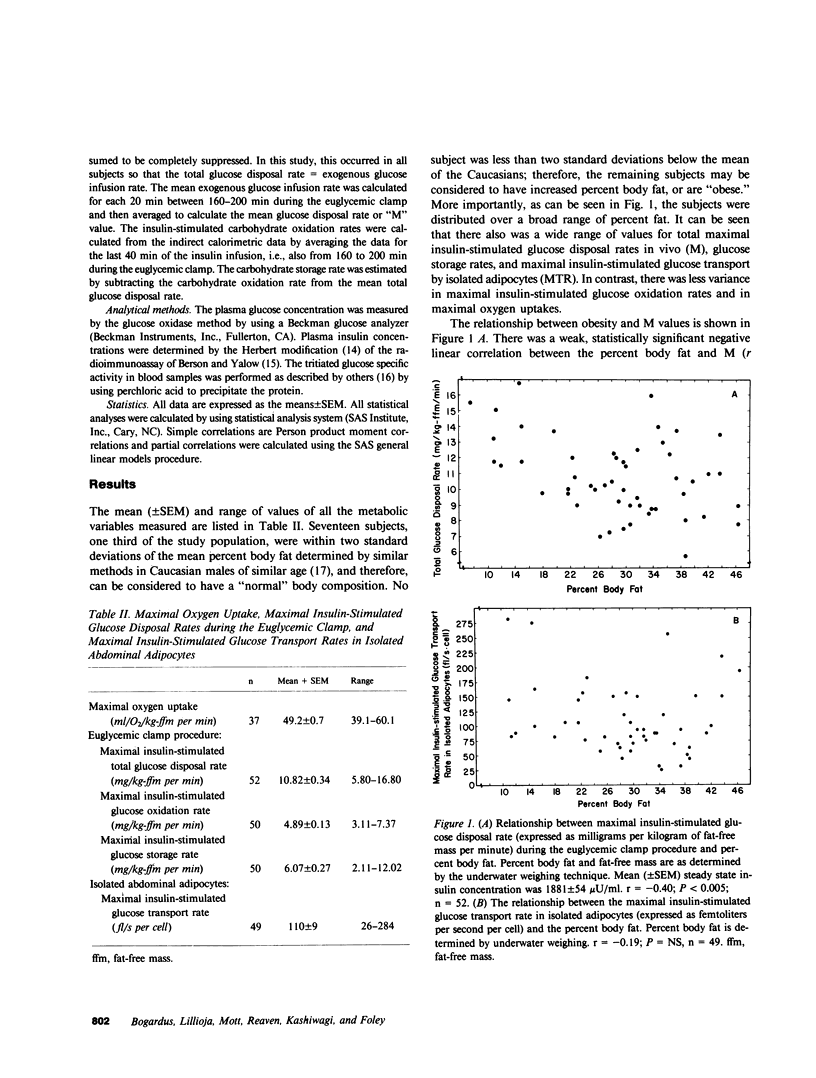
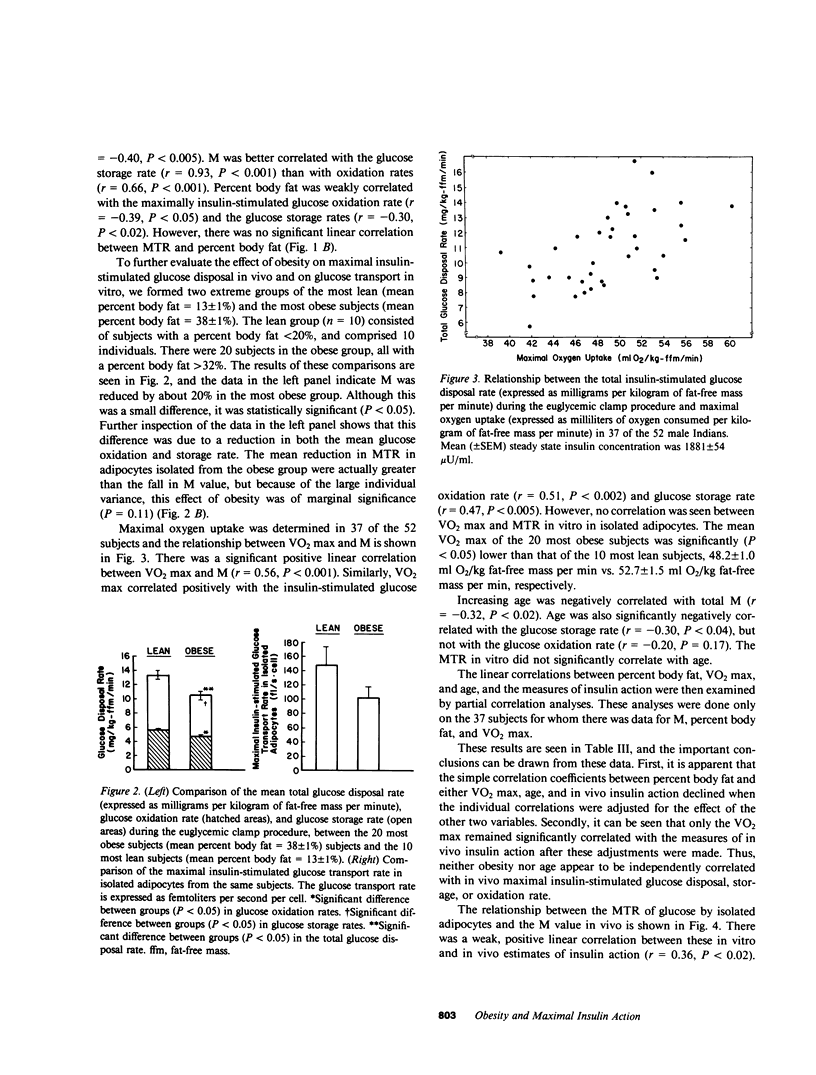
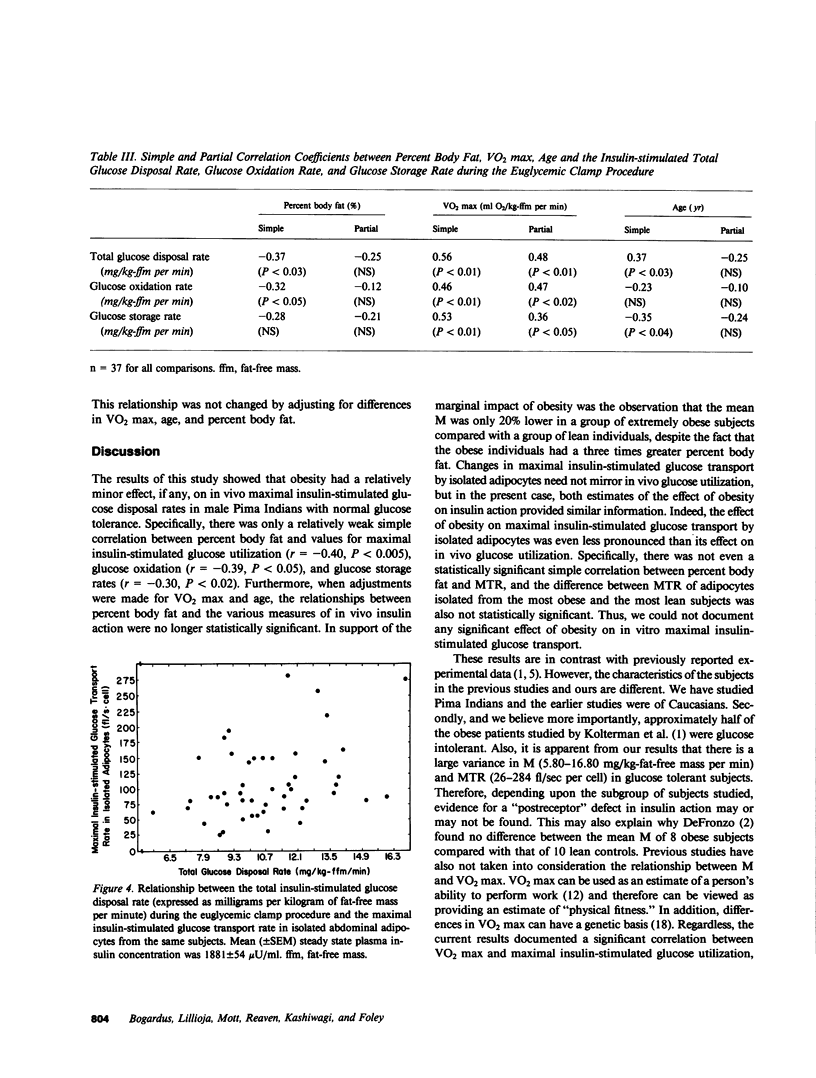
Previous studies have left unanswered whether human obesity, independent of glucose intolerance, is associated with a "postreceptor" defect in insulin action. We have studied the relationship between the degree of obesity (as estimated by underwater weighing) and the maximal insulin-stimulated glucose disposal rate (M) in vivo in 52 glucose-tolerant Pima Indian males. The relationship was examined independently of differences in age and maximal oxygen uptake (an estimate of "physical fitness"). The maximal insulin-stimulated glucose transport rate (MTR) was also measured in isolated abdominal adipocytes from the same subjects to determine whether differences in M could be explained by differences in glucose transport. The results showed that there was a large variance in M and MTR among these glucose-tolerant subjects. M was better correlated with glucose storage rates than with oxidation rates, as estimated by indirect calorimetry. The most obese subjects had only a 20% lower mean M and 30% lower MTR than the most lean subjects. The lower M in the obese subjects was due to both lower glucose oxidation and storage rates. There was no significant, independent correlation between age or degree of obesity and M or MTR. The maximal oxygen uptake (VO2 max) appeared to independently account for 20% of the variance observed in M. MTR was only weakly correlated with M (r = 0.36, P less than 0.02). We concluded that differences in M in these glucose-tolerant subjects must be explained by factor(s) other than maximal oxygen uptake, age, maximal insulin-stimulated glucose transport in vitro, or degree of adiposity per se.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Best J. D., Judzewitsch R. G., Pfeifer M. A., Beard J. C., Halter J. B., Porte D., Jr The effect of chronic sulfonylurea therapy on hepatic glucose production in non-insulin-dependent diabetes. Diabetes. 1982 Apr;31(4 Pt 1):333–338. doi: 10.2337/diab.31.4.333. [DOI] [PubMed] [Google Scholar]
- Chumlea W. C., Roche A. F., Siervogel R. M., Knittle J. L., Webb P. Adipocytes and adiposity in adults. Am J Clin Nutr. 1981 Sep;34(9):1798–1803. doi: 10.1093/ajcn/34.9.1798. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A. Insulin secretion, insulin resistance, and obesity. Int J Obes. 1982;6 (Suppl 1):73–82. [PubMed] [Google Scholar]
- Defronzo R. A. Glucose intolerance and aging: evidence for tissue insensitivity to insulin. Diabetes. 1979 Dec;28(12):1095–1101. doi: 10.2337/diab.28.12.1095. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Kashiwagi A., Verso M. A., Andrews J., Vasquez B., Reaven G., Foley J. E. In vitro insulin resistance of human adipocytes isolated from subjects with noninsulin-dependent diabetes mellitus. J Clin Invest. 1983 Oct;72(4):1246–1254. doi: 10.1172/JCI111080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klissouras V. Heritability of adaptive variation. J Appl Physiol. 1971 Sep;31(3):338–344. doi: 10.1152/jappl.1971.31.3.338. [DOI] [PubMed] [Google Scholar]
- Kolterman O. G., Insel J., Saekow M., Olefsky J. M. Mechanisms of insulin resistance in human obesity: evidence for receptor and postreceptor defects. J Clin Invest. 1980 Jun;65(6):1272–1284. doi: 10.1172/JCI109790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am J Physiol. 1981 Jun;240(6):E630–E639. doi: 10.1152/ajpendo.1981.240.6.E630. [DOI] [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Scarlett J. A., Gray R. S., Griffin J., Olefsky J. M., Kolterman O. G. Insulin treatment reverses the insulin resistance of type II diabetes mellitus. Diabetes Care. 1982 Jul-Aug;5(4):353–363. doi: 10.2337/diacare.5.4.353. [DOI] [PubMed] [Google Scholar]
- Shephard R. J., Allen C., Benade A. J., Davies C. T., Di Prampero P. E., Hedman R., Merriman J. E., Myhre K., Simmons R. The maximum oxygen intake. An international reference standard of cardiorespiratory fitness. Bull World Health Organ. 1968;38(5):757–764. [PMC free article] [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960 Jul;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]


