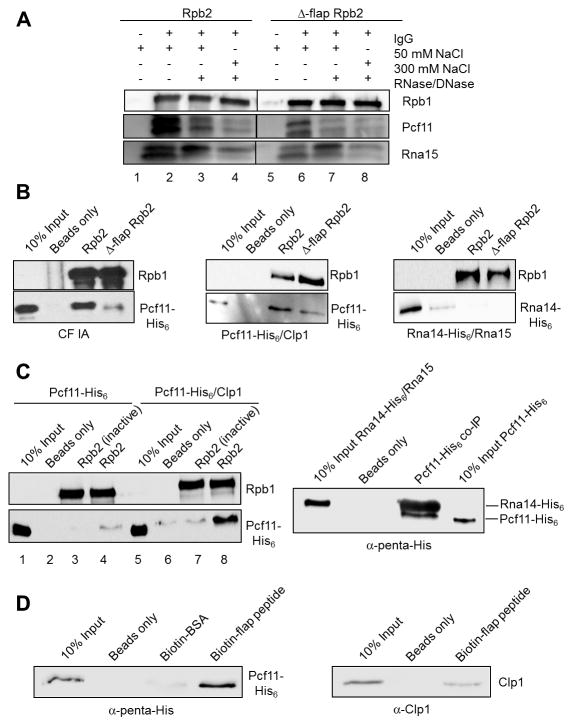
Figure 4. CF IA makes direct contact with the Pol II flap loop.
(A) Western blot of CF IA subunits Pcf11 and Rna15 co-immunoprecipitated with Wt and Δ-flap Pol II from extract using the TAP tag on Rpb2. As indicated, precipitates were treated with 50 or 300 mM NaCl washes or with an RNase/DNase mixture before elution. The samples were loaded onto the same gel, and the intervening lanes were removed as demarked by the line between the Wt and the mutant fractions.
(B) Western blots of recombinant CF IA (left panels), recombinant Pcf11/Clp1 heterodimer (middle panels) and recombinant Rna14/Rna15 heterodimer (right panels) co-immunoprecipitated with TAP-purified Wt and Δ-flap Pol II and detected with His6 antibody.
(C) Western blots of recombinant Pcf11 and recombinant Pcf11/Clp1 heterodimer co-immunoprecipitated with TAP-purified Wt Pol II (left panels) and of recombinant Pcf11 co-immunoprecipitated with recombinant Rna14/Rna15 heterodimer (right panel). In the experiment shown in the left panels, ”Rbp2 (inactive)” indicates Pol II immunoprecipitated from transcriptionally inactive extract. In the experiment shown in the right panel, a rabbit polyclonal anti-Rna15 antibody was used to immunoprecipitate Rna14/Rna15 pre-incubated with Pcf11 and Rna14 and Pcf11 detected by antibody against the His6 tag on both proteins.
(D) Western blots of recombinant Pcf11/Clp1 heterodimer detected with an anti-penta-His antibody (left panel) and recombinant Clp1 detected with a rabbit polyclonal anti-Clp1 antibody (right panel) co-immunoprecipitated with biotinylated flap loop peptide.
All co-immunoprecipitation experiments were performed two-four times each.